Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Stoich
front 1 What amount of oxygen, O2, (in moles) contains 1.8×10^22 molecules?
| back 1 B |
front 2 Which compound has the empirical formula with the greatest mass?
| back 2 B |
front 3 __C2H2(g) + __O2(g) → __ CO2(g) + __ H2O(g)
| back 3 D |
front 4 3.0 dm3 of sulfur dioxide is reacted with 2.0 dm3 of oxygen according to the equation below. 2SO2(g) + O2(g) → 2SO3(g)
| back 4 C |
front 5 What will happen to the volume of a fixed mass of gas when its pressure and temperature (in Kelvin) are both doubled?
| back 5 A |
front 6 What volume (in dm3) of 0.30 mol dm–3 NaCl solution can be prepared from 0.060 mol of solute?
| back 6 B |
front 7 Ag(s) + NO3–(aq) + H+(aq) Ag+(aq) + NO(g) + H2O(l)
| back 7 D |
front 8 What amount (in moles) is present in 2.0 g of sodium hydroxide, NaOH?
| back 8 A |
front 9 A hydrocarbon contains 90% by mass of carbon. What is its empirical formula?
| back 9 B |
front 10 Copper can react with nitric acid as follows.
| back 10 C |
front 11 Lithium hydroxide reacts with carbon dioxide as follows. 2LiOH + CO2 → Li2 CO3 + H2O
| back 11 B |
front 12 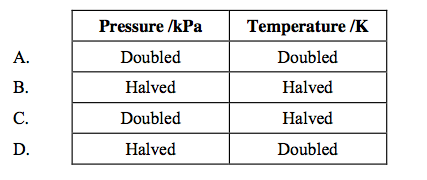 Which change in conditions would increase the volume of a fixed mass of gas? | back 12 D |
front 13 Which solution contains the smallest amount of H+ ions?
| back 13 A |
front 14 How many hydrogen atoms are contained in one mole of ethanol, C2H5OH?
| back 14 D |
front 15 The percentage by mass of the elements in a compound is
| back 15 B |
front 16 What is the coefficient for O2(g) when the equation below is balanced? __C3H8(g) + __O2(g) → __CO2(g) + __H2O(g)
| back 16 C |
front 17 What amount of NaCl (in moles) is required to prepare 250 cm3 of a 0.200 mol dm–3 solution?
| back 17 D |
front 18 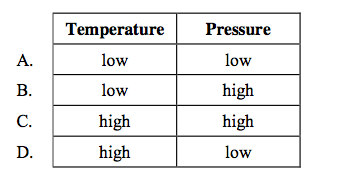 For which set of conditions does a fixed mass of an ideal gas have the greatest volume? | back 18 D |
front 19 Which of the following contains the greatest number of molecules?
| back 19 A |
front 20 Which of the following compounds has/have the empirical formula CH2O? I. CH3COOH II. C6H12O6 III. C12H22O11
| back 20 C |
front 21 Assuming complete reaction, what volume of 0.200 mol dm–3 HCl(aq) is required to neutralize 25.0 cm3 of 0.200 mol dm–3 Ba(OH)2(aq)?
| back 21 C |
front 22 Under what conditions would one mole of methane gas, CH4, occupy the smallest volume?
| back 22 B |
front 23 The temperature in Kelvin of 2.0 dm3 of an ideal gas is doubled and its pressure is increased by a factor of four. What is the final volume of the gas?
| back 23 A |
front 24 Consider the following equation.
| back 24 A |
front 25 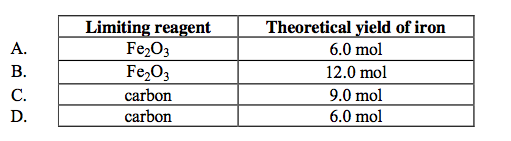 6.0 moles of Fe2O3(s) reacts with 9.0 moles of carbon in a blast furnace according to the equation below.
| back 25 D |
front 26 What volume of 0.500 mol dm–3 HCl(aq) is required to react completely with 10.0 g of calcium carbonate according to the equation below?
| back 26 D |
front 27 Which is a correct definition of the term empirical formula?
| back 27 D |
front 28 The reaction of ethanal and oxygen can be represented by the unbalanced equation below. __ CH3CHO + __ O2 → __ CO2 + __ H2O
| back 28 C |
front 29 The equation for the complete combustion of butane is
| back 29 C |
front 30 Which solution contains the greatest amount (in mol) of solute?
| back 30 C |
front 31 A fixed mass of an ideal gas has a volume of 800 cm3 under certain conditions. The pressure (in kPa) and temperature (in K) are both doubled. What is the volume of the gas after these changes with other conditions remaining the same?
| back 31 B |
front 32 How many oxygen atoms are present in 0.0500 mol carbon dioxide?
| back 32 B |
front 33 The complete oxidation of propane produces carbon dioxide and water as shown below.
| back 33 B |
front 34 The relative molecular mass (Mr) of a compound is 60. Which formulas are possible for this compound?
| back 34 C |
front 35 35. Which sample has the least number of atoms?
| back 35 A |
front 36 Avogadro’s constant has the same value as the number of
| back 36 A |
front 37 Which aqueous solution contains the most hydrogen ions?
| back 37 A |
front 38 What is the total of all the coefficients in the balanced equation for the reduction of 1 mol of MnO4–?
| back 38 D |
front 39 Which contains the same number of ions as the value of Avogadro’s constant?
| back 39 A |
front 40 A reaction occurring in the extraction of lead from its ore can be represented by this unbalanced equation:
| back 40 C |
front 41 The equation for a reaction occurring in the synthesis of methanol is
| back 41 A |
front 42 Which solution contains 0.1 mol of sodium hydroxide?
| back 42 C |
front 43 A cylinder of gas is at a pressure of 40 kPa. The volume and temperature (in K) are both doubled. What is the pressure of the gas after these changes?
| back 43 C |
front 44 Which of the following quantities has units?
| back 44 C |
front 45 What is the purpose of the beam of high energy electrons used in a mass spectrometer?
| back 45 A |
front 46 The empirical formula of a compound is C2H4O. Which molecular formulas are possible for this compound?
| back 46 C |
front 47 Calcium carbonate decomposes on heating as shown below. CaCO3 CaO + CO2
| back 47 B |
front 48 Sodium reacts with water as shown below.
| back 48 D |
front 49 What is the total number of ions present in the formula, Al2(SO4)3?
| back 49 C |
front 50 A 4 g sample of sodium hydroxide, NaOH, is dissolved in water and made up to 500 cm3 of aqueous solution. What is the concentration of the resulting solution?
| back 50 B |
front 51 Methane, CH4, burns in oxygen gas to form carbon dioxide and water. How many moles of carbon dioxide will be formed from 8.0 g of methane?
| back 51 B |
front 52 What is the empirical formula of a compound containing 50% by mass of element X (Ar = 20)
| back 52 D |
front 53 Assuming complete reaction, what volume of 0.200 mol dm–3 potassium hydroxide solution (KOH(aq)), is required to neutralize 25.0 cm3 of 0.200 mol dm–3 aqueous sulfuric acid,
| back 53 C |
front 54 Consider the following reaction.
| back 54 A |
front 55 The temperature in Kelvin of 1.0 dm3 of an ideal gas is doubled and its pressure is tripled. What is the final volume of the gas in dm3?
| back 55 B |
front 56 On complete combustion, a sample of a hydrocarbon compound produces 1.5 mol of carbon
| back 56 D |
front 57 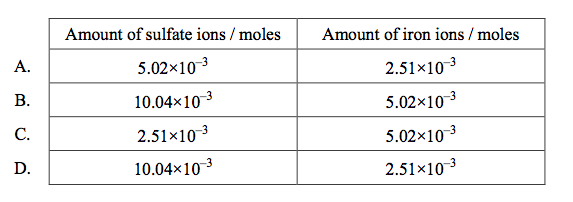 When excess BaCl2(aq) was added to a sample of Fe(NH4)2(SO4)2(aq) to determine the amount in moles of sulfate present, 5.02×10–3 mol of BaSO4 was obtained. How many moles of sulfate
| back 57 A |
front 58 What volume of 0.500 mol dm–3 sulfuric acid solution is required to react completely with 10.0 g of calcium carbonate according to the equation below?
| back 58 B |
front 59 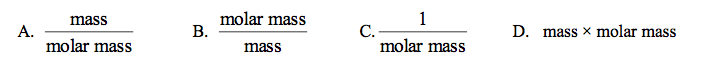 Which expression gives the amount (in mol) of a substance, if the mass is given in grams? | back 59 A |
front 60 What is the total number of atoms in 0.20 mol of propanone, CH3COCH3?
| back 60 C |
front 61 When the equation below is balanced for 1 mol of C3H4, what is the coefficient for O2?
| back 61 C |
front 62 Ethyne, C2H2, reacts with oxygen according to the equation below. What volume of oxygen (in dm3) reacts with 0.40 dm3 of C2H2?
| back 62 C |
front 63 Ethyne, C2H2, reacts with oxygen according to the equation below. What volume of oxygen (in dm3) reacts with 0.40 dm3 of C2H2?
| back 63 C |
front 64 What is the coefficient for H+ when the redox equation below is balanced?
| back 64 D |
front 65 How many hydrogen atoms are in one mole of ethanol, C2H5OH?
| back 65 B |
front 66 What is the coefficient for H2SO4(aq) when the following equation is balanced, using the smallest possible integers?
| back 66 C |
front 67 Air bags in cars inflate when sodium azide decomposes to form sodium and nitrogen: 2NaN3(s) → 2Na(s) + 3N2(g)
| back 67 C |
front 68 What volume, in cm3, of 0.200 mol dm–3 HCl(aq) is required to neutralize 25.0 cm3 of 0.200 mol dm–3 Ba(OH)2(aq)?
| back 68 C |