Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Microbiology Exam 2
front 1 CHAPTER 18 | back 1 Diagnosing Infections |
front 2 What are the three categories of methods to identify unknown bacteria? | back 2 Phenotypic, Immunological, and Genotypic |
front 3 What is phenotypic testing? | back 3 Observation of microbe’s microscopic and macroscopic morphology, physiology, antimicrobial susceptibility, and biochemical properties |
front 4 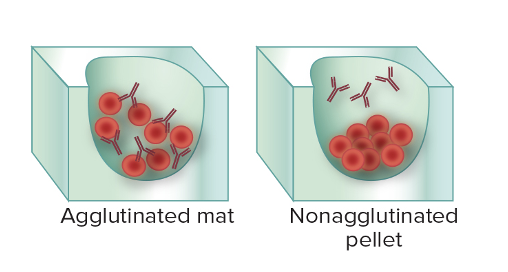 What is immunological testing? | back 4 Analysis of microbe using antibodies or of patients’ antibodies using prepackaged antigens |
front 5  Genotypic | back 5 Analysis of microbe’s DNA or RNA |
front 6 What are the different sample sites for collection? | back 6 Saliva, Sputum (thick stuff from a hacking cough), swab - Blood - Urine (through catheter or clean catch) - Skin (swab) - Spinal tap (CSF) - Feces - Vaginal swab or stick or Penis swab/stick - Skin (scalpel) |
front 7 What is a culturette? | back 7 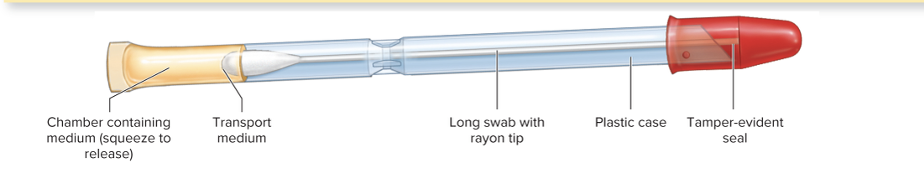 A sterile transport swab with a carrier |
front 8 What happens once you collect a specimen? | back 8 It's promptly transported to a LAB, stored appropriately (usually in the fridge) - Special swab and transport systems can be used to maintain it in a stable condition for hour - May have nonnutritive maintenance media, buffering system, or anaerobic environment to prevent the destruction of O2 sensitive bacteria. |
front 9 Phenotypical method relates to ____________. | back 9 Expression of genes that is UNIQUE to that particular microbe Phenotypic testing is based on the expression of a microbe’s genes, which lets us observe what the organism is actually doing — such as how it stains, how it grows or reacts on different media, and what its cell shape and structure (morphology) look like. |
front 10 How is a specimen cultivated, and what are the two different medias that can be used? | back 10 After a specimen (like blood, urine, or a throat swab) is collected, cultivation means growing and multiplying any microorganisms that might be present in that sample under controlled lab conditions. This can mean using specialized media that reveals identifying characteristics such as colony appearance, motility, and gas requirements. - Selective media: encourage growth of only one pathogen and used to enrich specimen. - Differential: identify definitive characteristics and fermentation patters (bacteria itself is NOT changing, but the plate is changing) In phenotypic testing, we’re not changing the bacteria itself (its genetics or inherent traits) — instead, we’re changing the environment, like the type of plate or media the bacteria is grown on. For example:
|
front 11 What are hemolysins? | back 11 Enzymes that lyse RBCs to release iron-rich hemoglobin so bacteria is able to multiply. Beta, Alpha, and Gamma. Some microbes like to extract nutrients from RBCs. |
front 12 What is a dichotomous key? | back 12 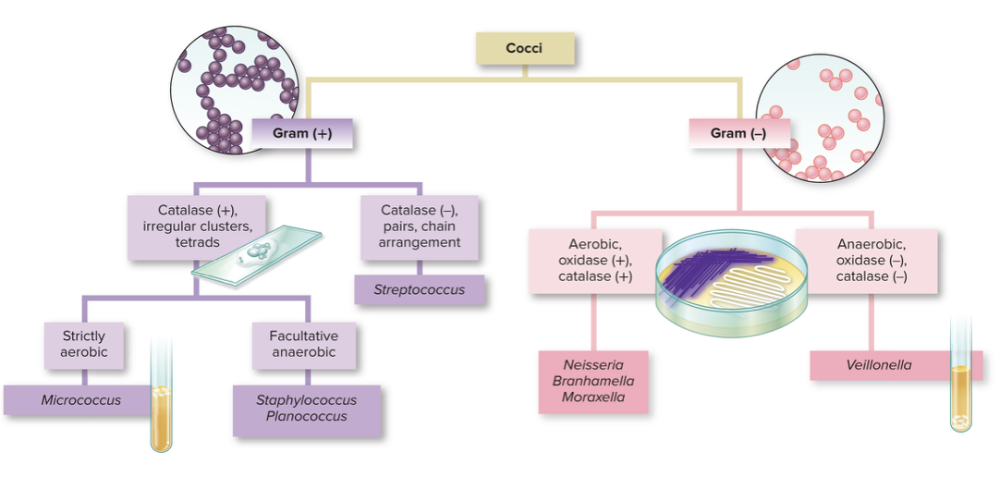 Graphic method that essentially a flowchart leading to the identification of specimens. It allows you graph all the way down to a particular microbe. |
front 13 What is biochemical testing? | back 13 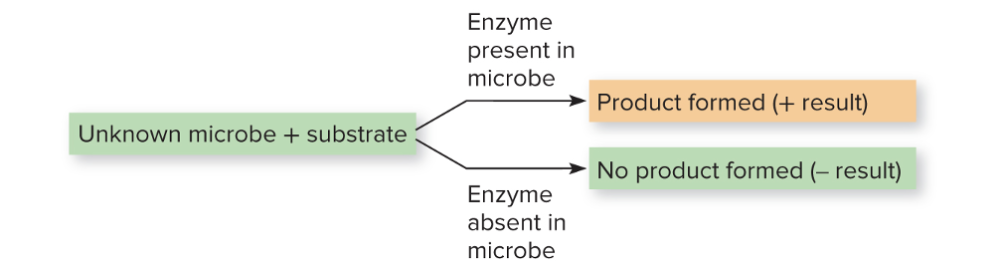 Physiological reactions (physical change like color, bubbling, shape) of bacteria to nutrients and a special substrates provide indirect evidence of enzyme systems. This can be done manually or by machines. - These tests are based on enzyme-mediated metabolic reactions, and usually visualized by a color change. If you have a unknown microbe and a substrate, if there's product, the enzyme is there (and that microbe) No product, then the enzyme is not there, and it lacks the enzyme that can utilize that substrate. |
front 14 Explain the connection in biochemical testing between substrate, enzyme, and final product. | back 14 Biochemical testing is all about seeing what enzymes the bacteria have and how those enzymes react with specific substrates. Think of it like giving the bacteria a little “quiz”:
All those reactions together form a kind of metabolic profile, which scientists use to identify the microbe. |
front 15 What is antimicrobial susceptibility testing? | back 15 This is used in determining drugs to be used in treatment. Most of the atuomated phenotypic systems incorporate a panel of commonly used antimicrobials for the particular infection site and stimultaneously test susceptibility while identifying the pathogen
And yes |
front 16 What is phage testing? | back 16 Involves inoculating a lawn of cells onto agar in a Petri dish, mapping off blocks, and applying a different phage to each sectioned area of growth. Cleared areas corresponding to lysed cells indicate sensitivity to that phage. - Involves viruses that attack bacteria in species-specific and strain specific ways. - Used for tracing bacterial strains in epidemics. Here’s how it works step-by-step:
|
front 17 What microbe is easy to identify with phage testing? | back 17 Salmonella If bacteria has specific receptors that phage is looking for THEN it can get inside. In other words, very particular phages will recognize bacteria and get inside bacteria via recognition of expression of proteins or receptors. If bacteria does not correct proteins for virus to adsorb too, then it will NOT bind to and cause cell death All about the expression of proteins that allow the virus to get in, and kill bacteria cell |
front 18 How do you determine the clinical significance of cultures? | back 18 It is important to recognize if an isolated colony is clinically important (is there an infection?) or merely a contaminant or normal biota. - Focus on the number of microbes (ex. few colonies of E. coli can be normal biota but a hundred colonies would mean an active infection). Sometimes, a single colony might mean an infection if it's not normal biota. Ex. With mycobacterium tuberculosis, even finding just one colony of a known disease-causing organism (a true pathogen), or an opportunist in a place that should be completely sterile, is a strong indication that it’s causing an infection. |
front 19 What are the drawbacks of phenotypic methods? | back 19 Culturing microbes takes a minimum of 18-24 hrs (often longer) Many infectious conditions can be caused by non-culturable organisms, leaving a wide range of chance that the organism we do culture is a bystander. |
front 20 What are the five different types of phenotypic testing? Which one DOES NOT require cultivation? | back 20 MS DSB - Master's in DSB - Miscellaneous (Phage typing, cell culture growth, and animal inoculation)
- Susceptibility testing: a particular pattern of antimicrobial susceptibilities can lead to the identity of a microbe - Direct examination (NO CULTIVATION): Microscopy of patient specimens, usually after staining - Selective/differential growth: Use of specialized media that reveal identifying characteristics such as colony appearance, motility, and gas requirements - Biochemical testing: Growth of microbe in media that detects the presence of microbe’s enzymes, creating a metabolic fingerprint |
front 21 In immunological methods, what is serology? | back 21 Serology is the diagnostic testing of blood serum (the clear part of your blood) to detect antibodies or antigens related to infections
|
front 22 With the same techniques used in serology, we can also detect immune markers in what other specimens? | back 22 Sera, Urine, CSF, whole tissues, saliva |
front 23 What are the two basic sides towards serological testing? | back 23 First scenario: In patient's serum, the antibody is UNKNOWN. We added prepared microbial agent. If it binds, then it tells you that the person has the antibody (agglutination) - tests if a person has antibodies (immunity/exposure). Second scenario: You have an isolated colony of unknown microbes. But you have antibodies of a known identify. When you combine them, if they bind, you can tell (agglutinations), if they don't, you know it's not that microbe. - identifies the specific bacterium or strain. |
front 24 The presence of antibodies in someone's system can show what things? | back 24 They had the infection, or they have a vaccine against it |
front 25 What are the 7 different types of immunological diagnostic methods? | back 25 PIA is a WIFE (acronym) - Precipitation: Smaller complexes of antibody–antigen. - Immunochromatography: Most common form is a lateral flow system, supplied in prepackaged cartridges that produce a colored stripe. - Agglutination: Antibody-mediated clumping of whole cells. - Western Blot: Electrophoresis separates proteins (either antigens or antibodies) and then labeled antibodies or antigens are used for detection. - In vivo tests: Antigen introduced into a patient to elicit a reaction as in TB skin test. - Fluorescent antibodies:
- ELISA: Sandwiching technique conducted in microwells using Ag, Ab, and a secondary Ab to produce a color change. ** note even if there's a color change, it is still this because it can happen due to binding between antibody and antigen. |
front 26 True or False:
| back 26 FALSE But,
|
front 27 What are the three essential differences between agglutination and precipitation? | back 27 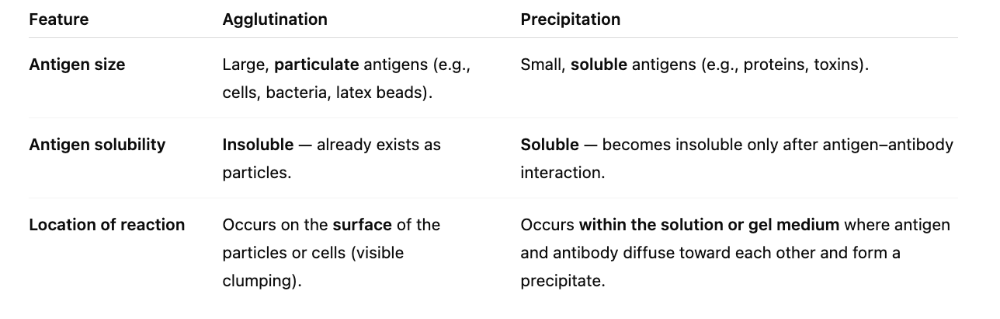 1. Size 2. Solubility 3. Location of antigen |
front 28 What is the difference between agglutination and precipitation? | back 28 In both reactions, antigen is interlinked by several antibodies to form insoluble clumps that settle out of solution. Mostly, it's the parts of the antigen vs. whole antigen. In agglutination, antigens are whole cells such as RBCs, bacteria, or viruses displaying surface antigens. This is seen MORE easily because of visible clumps.
Precipitation: antigen is a soluble molecule. |
front 29 What common test is agglutination used for? | back 29 Determining blood compatibility. |
front 30 What is immunochromatography? | back 30 - Lateral flow test - pregnancy, COVID-19 tests - This is a plastic cartilage that directs fluid flow in one direction, where it will encounter antibodies. - When the antigen encounters the antibodies, there is a color change. |
front 31 What are the steps to a lateral flow test? | back 31
|
front 32 What is an antibody titer? | back 32 - The concentration of antibodies in a sample. This tells us the minimum amount of antibody needed to react with the antigen. - Antibody titer measures the concentration of antibodies in a patient’s serum by serially diluting it with a known antigen and identifying the highest dilution that still produces agglutination, helping diagnose past infections, immunity, or autoimmune disorders. |
front 33 For bacteria, what is the difference between serotypes and serotyping? | back 33
|
front 34 What is a western blot test? | back 34
|
front 35 What do you do know a western blot test? | back 35 In a Western blot, the lab knows the antigens — they are the proteins from the pathogen that are separated and immobilized on the membrane.
|
front 36 How many antibodies are used in western blot? | back 36 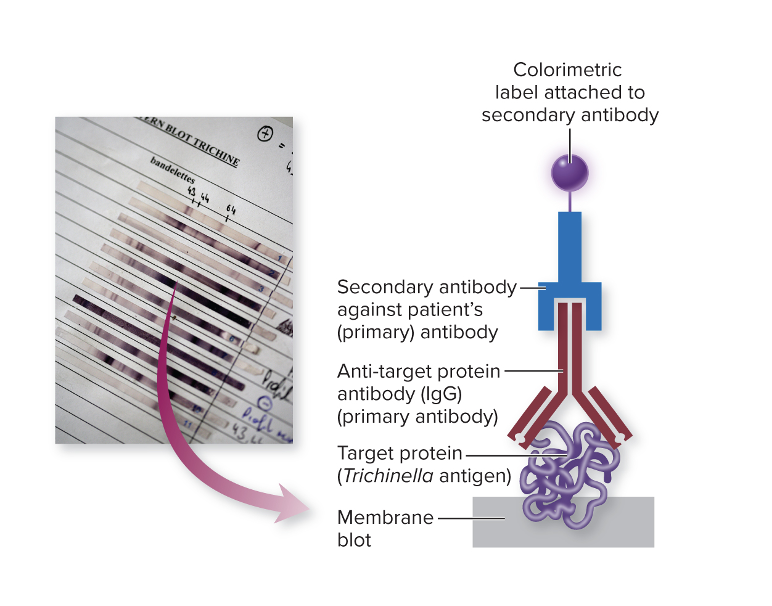 In this setup, there are usually two different antibodies involved, each with a specific role:
|
front 37 What are the two types of immunofluorescence testing? | back 37 1. Indirect Testing
Main idea: Indirect IFA detects unknown antibodies in patient serum by using a fluorescent-labeled secondary antibody to visualize them bound to a known antigen. 2. Direct Testing
|
front 38 What is known and not known between indirect and direct testing? | back 38 Direct Immunofluorescence (DIF)
Indirect Immunofluorescence (IFA)
|
front 39 What's the difference between Indirect Testing and Western Blot? | back 39
|
front 40 What is ELISA? | back 40 Enzyme-Linked Immunosorbent Assay (ELISA)
|
front 41 What's the difference between Direct, Indirect and Sandwich ELISA? | back 41 1. Direct ELISA
2. Indirect ELISA
3. Sandwich ELISA
|
front 42 What is Indirect ELISA? | back 42 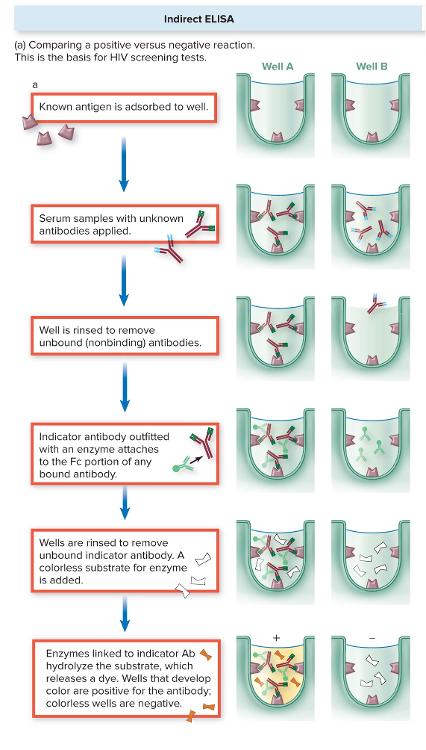
___________________________________
|
front 43 What is the sandwhich ELISA? | back 43 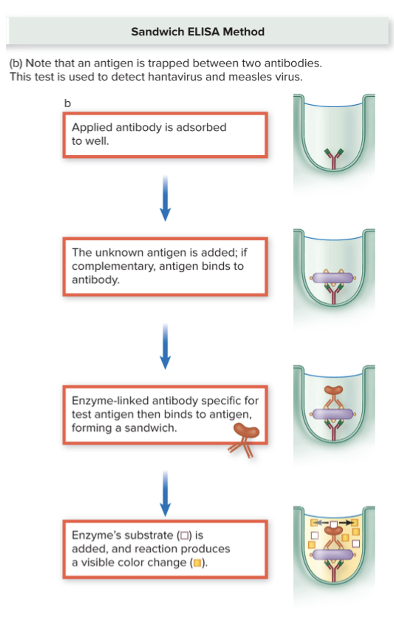 Two antibodies.
|
front 44 What is the goal of each of the ELISAs | back 44 1. Direct ELISA
2. Indirect ELISA
3. Sandwich ELISA
|
front 45 What is the complement fixation test? | back 45 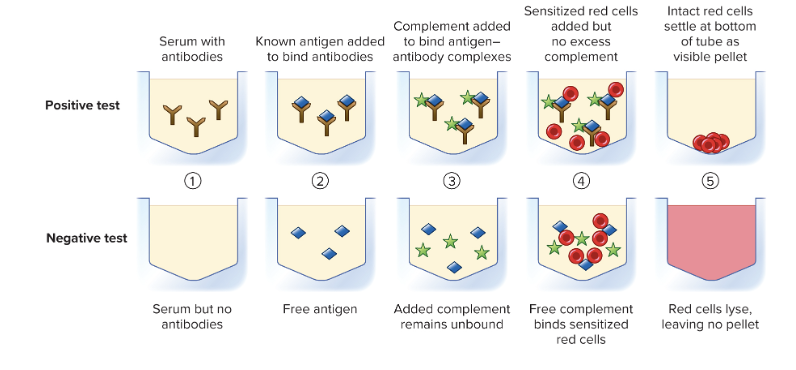 Many viral and fungal diseases are diagnosed using the principle that complement can lyse red blood cells KNOWN: antigen unknown: patient's antibodies
|
front 46 What is in vivo testing? | back 46 It is similar to serological testing, but an antigen or antibody is introduced into a patient to elicit some sort of visible reaction (welt or inflammation) Ex.
|
front 47 What is specificity? | back 47
|
front 48 A test with high specificity will have _________. | back 48 Low false positives (no positive test unless the exact antigen-antibody complex is present). |
front 49 What is sensitivity? | back 49
|
front 50 A test with high sensitivity will have _____________. | back 50 low false-negative rate
|
front 51 What are the three types of genotypic testing? | back 51 Anything dealing with nucleic acids! WHY N? 1. Nucleic acid amplification tests: using primers to amplify specific DNA sequences. (PCR, TMA, other NAAT) 2. Hybridization: FISH (in situ) 3. Whole-genome sequencing: high-throughout methods have made whole-genome sequencing widely accessible. |
front 52 What is PCR? | back 52 - Results in the production of numerous copies of DNA or RNA molecules within hours - Very sensitive, even amplify small amounts of nucleic acids in sample.
- the purpose of amplification in PCR is to make millions of copies of a specific DNA segment so it can be easily detected, studied, or analyzed.
|
front 53 What does PCR require? | back 53
|
front 54 What are the different types of PCR? | back 54 1. Real-Time PCR (qPCR)
2. Reverse-Transcriptase PCR (RT-PCR)
3. Multiplex PCR
4. Panbacterial qPCR
|
front 55 What is hybridization? | back 55
|
front 56 What is FISH? | back 56
|
front 57 What is the difference between FISH and hybridization? | back 57 Regular hybridization:
FISH (Fluorescence In Situ Hybridization):
|
front 58 Name two examples of techniques that employ hybridization. | back 58
|
front 59 What are the materials required for FISH? | back 59
|
front 60 What is whole genome sequencing? | back 60
|
front 61 What is a microarray? | back 61 What they are:
How they work:
Main use:
|
front 62 Chapter 12 | back 62 Antimicrobial Treatment |
front 63 What is the goal of antimicrobial therapy? | back 63 Administer a drug to an infected person that destroys the INFECTIOUS agent without harming the host cells! |
front 64 What are some characteristics to the ideal antimicrobial drug | back 64
|
front 65 What is prophylaxis? | back 65 Use of a drug to prevent imminent infection of a person at high risk Prophylaxis means preventing a disease before it happens |
front 66 What is antimicrobial chemotherapy? | back 66 The use of drugs to control infection (specifically chemicals) |
front 67 What is antimicrobials? | back 67 All-inclusive term for any antimicrobial drug, regardless of what type of microorganism it targets |
front 68 Antibiotics can be produced ________ or created ___________. | back 68 by the natural metabolic processes (natural sources) of some microorganisms or created by scientists. |
front 69 What's more effective: semisynthetic drugs or synthetic drugs? | back 69
|
front 70 What's the difference between narrow-spectrum and broad-spectrum antimicrobials? | back 70
|
front 71 What is the most common origin of antimicrobial drugs? | back 71 Antibiotics are common metabolic products of bacteria and fungi.
|
front 72 What are the three factors that must be known before starting antimicrobial treat? | back 72 1. What is the identity of the microorganism? - many methods 2. What is the microorganism's susceptibility to various drugs? (can it be harmed or hindered) 3. How does the patient react to the drug? |
front 73 What is the Kirby-Bauer Technique? | back 73 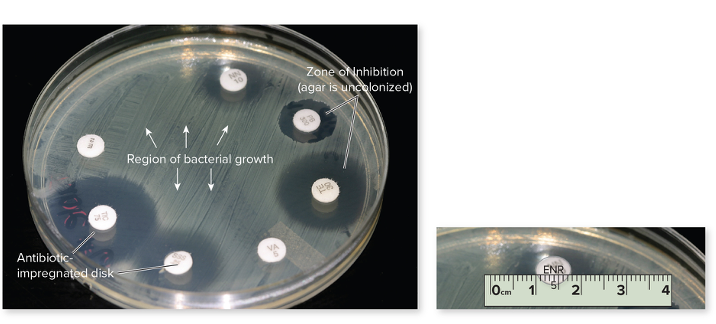 The Kirby-Bauer test measures how well an antibiotic can stop bacterial growth. Small discs containing antibiotics (pre-measured amount) are placed on a plate with bacteria (special medium), and the drug spreads outward. If the antibiotic works, it creates a zone of inhibition (edge to edge of no growth) — a clear area where bacteria can’t grow. The size of this zone shows how sensitive or resistant the bacteria are to that antibiotic. It will then be compared with the standard for each drug, determining if its sensitive or resistant or in the middle. |
front 74 What is the Etest? | back 74 It's an alternative to Kirby-Bauer. The E-test is used to determine the minimum inhibitory concentration (MIC) — the smallest amount of an antibiotic needed to stop bacterial growth. A plastic strip with a predefined gradient of antibiotic concentrations is placed on an agar plate that has been spread with bacteria. As the antibiotic diffuses from the strip, it creates zones where bacteria cannot grow. The point where bacterial growth stops along the strip shows the MIC value, indicating how sensitive the bacteria are to that antibiotic. From bottom to top, the concentration of antibiotic increases as you go up the strip. Instead of a perfect circle around a disc, the E-test creates an elliptical (oval-shaped) zone of inhibition that follows the antibiotic gradient on the strip. The point where the edge of bacterial growth meets the strip marks the minimum inhibitory concentration (MIC) — the lowest antibiotic concentration that prevents growth. |
front 75 What's the difference between E-test and Kirby-Bauer? | back 75 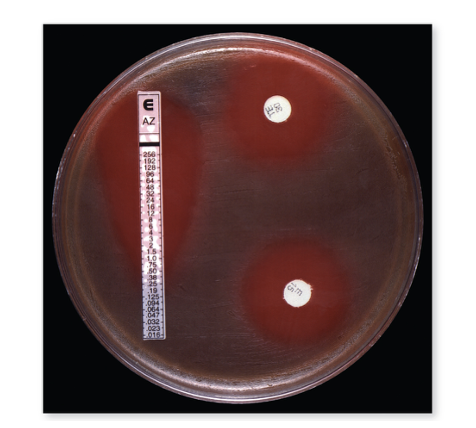
|
front 76 What's the purpose of an MIC? | back 76
|
front 77 What is the tube dilution test? | back 77 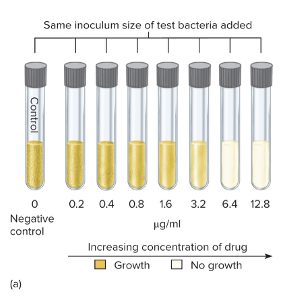 The tube dilution test is used to determine the minimum inhibitory concentration (MIC) — the lowest concentration of an antibiotic that prevents bacterial growth. Here’s how it works:
So basically:
|
front 78 Why might an antimicrobial treatment fail? | back 78 - It didn't get to the site of the infection - Resistant microbes - An infection may be caused by one or more pathogen, some which may be resistant |
front 79 What is the Therapeutic index (TI)? | back 79 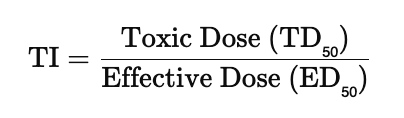 The ratio of the dose of the drug that is toxic to humans to its effective dose. The therapeutic index (TI) measures a drug’s safety margin — it’s the ratio between the toxic dose and the effective dose. A high TI means the drug is safer (there’s a big gap between helpful and harmful doses), while a low TI means it’s riskier because the effective and toxic doses are close together. TI of 1.1 is riskier than TI of 10. |
front 80 What is therapeutic window? | back 80
|
front 81 True or False: So, the lower the therapeutic index, the greater the margin of safety for that drug. | back 81  FALSE, it should be higher... So, the higher the therapeutic index, the greater the margin of safety for that drug. |
front 82 What is selective toxicity? | back 82 - Antimicrobial treatment drugs should kill or inhibit ONLY microbial cells without also damaging host tissues. Best drugs block actions or synthesis of microbes but not vertebrae cells. Ex. peniciliin - excellent selective toxicity, only blocks synthesis of cell wall in bacteria. |
front 83 What are the main 5 different mechanisms of metabolic targets on therapeutic agents? | back 83 1. Inhibition of cell wall synthesis 2. Inhibition of nucleic acid structure and function 3. Inhibition of protein synthesis, involving mainly ribosomes 4. Interfering with cell membrane structure or function 5. Inhibition of folic acid synthesis |
front 84 Why is folic acid important to microbes? | back 84 Folic acid (vitamin B₉) is super important to microbes because it’s essential for making DNA, RNA, and amino acids
|
front 85 Primary sites of antimicrobial treatment on bacterial cells! | back 85 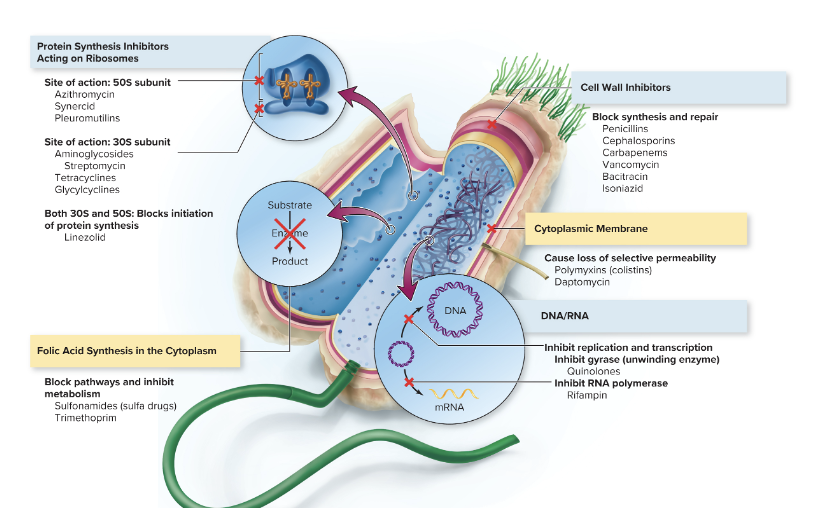 |
front 86 What are the three main effects or side effects of antimicrobials? | back 86 1. Toxicity to human organs 2. Human Allergic response to drugs (drugs can act as antigens) - Penicillin, then Sulfonamides 3. Altercation or Supression of Human Microbiota |
front 87 What is superinfection? | back 87 After an antimicrobial destroys beneficial resident species, other microbes that were once in small numbers can begin to overgrow and cause disease Ex.
a superinfection happens when antibiotics kill off the normal, helpful microbes in your body — like the ones in your gut or on your skin — and this gives opportunistic pathogens (like Candida or C. difficile) a chance to overgrow. Basically, the antibiotic wipes out the good guys, and the bad ones take over, causing a new infection on top of the original one you were treating. oth Candida albicans (a yeast) and Clostridioides difficile (a bacterium) normally live in small amounts in your body — Candida in places like your mouth, gut, and vagina, and C. difficile in your intestines. They usually don’t cause problems because your normal microbiota keep them in check. But when antibiotics kill off that good bacteria balance, these guys can overgrow — leading to infections |
front 88 What is the main example of broad-spectrum drugs? | back 88
|
front 89 What are the two examples of narrow-spectrum drugs? (PP) | back 89
|
front 90 What are the drugs targeting the cell wall? (PVCC) | back 90 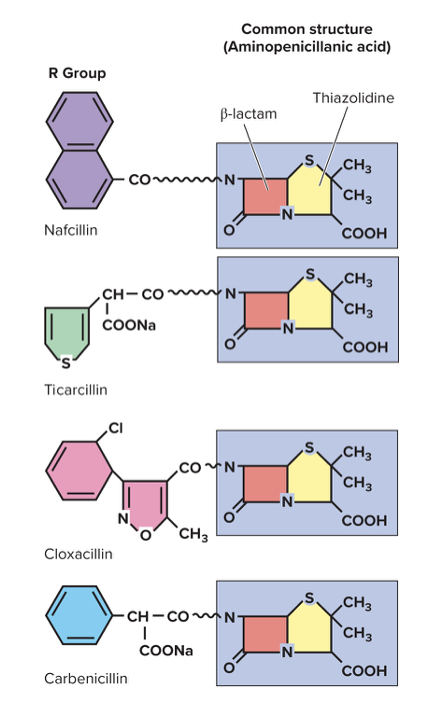 1. Penicillin
2. Cephalosporin (used when penicillin is not effective)
3. Carbapenem
4. Other miscellaneous drugs
|
front 91 What is clavulanic acid, and what is it mechanism of action? | back 91 Inhibits β-lactamase enzymes and is added to other penicillins to increase their effectiveness in the presence of penicillinase-producing bacteria Some bacteria make an enzyme called β-lactamase (or penicillinase), which basically destroys the β-lactam ring in penicillin and makes the antibiotic useless. To stop that, scientists created β-lactamase inhibitors (like clavulanic acid). These inhibitors are added to penicillins — for example, in Augmentin (amoxicillin + clavulanic acid). The inhibitor’s job is to bind to and block the β-lactamase enzyme, so the enzyme can’t destroy the penicillin. That way, the antibiotic can still attack the bacterial cell wall effectively |
front 92 What is vancomyocin, and what is the mechanism of action? | back 92 Targets the Cell Wall
|
front 93 What are the drugs targeting protein synthesis (impairs prokaryotic ribosomal function)? | back 93 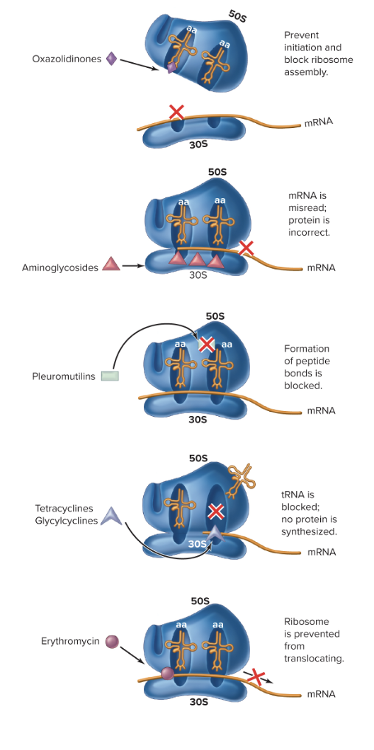 1. Aminoglycosides = "awful translation)
2. Oxazolidinoes
3. Pleuromutilins
4. Tetracyclines/Glycacyclines
5. Macrolides
|
front 94 What is streptomycin? | back 94 Inhibits proper protein synthesis
|
front 95 What is erythromycin? | back 95 Macrolide Antibiotic - inhibits protein synthesis as it inhibits translocation of subunit during translation - Can be used to treat mycobacterium infections in AIDS patients
|
front 96 What are antibacterial drugs targeting folic acid synthesis? | back 96 They prevent proper metabolism, preventing microbes from replicating, then not allowing them to make proteins, and this class can be used with other drugs. - Sulfa drugs or sulfonaminds
|
front 97 What are the antibacterial drugs targeting DNA or RNA? | back 97 1. Fluoroquinones (Ciprofloxacin, Levofloxacin, Nalidxic Acid, Trovafloxacin)
2. Ansamycin
|
front 98 What are the antibacterial drugs targeting cell membranes? | back 98 1. Polymyxins
- Daptomycin (subgroup) |
front 99 What is daptomycin? | back 99 - Type of polymxin (disrupts cell membrane)
|
front 100 What are three treatments for quorom-sensing pathways? | back 100
1. Interrupt quorum-sensing pathways 2. Daptomycin 3. Pretreatment |
front 101 Which of the following are treatments for helminths?
| back 101
|
front 102 What are three major modes of action for antiviral agents? - MPM | back 102 STOP penetration (virus into host cell), multiplication (replication, transcription, translation of viral molecules), and maturation of viral particles |
front 103 Explain the inhibition of virus entry? | back 103 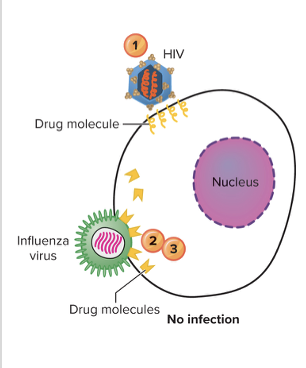 Receptor/Fusion/Uncoating Inhibitors
|
front 104 Explain inhibition of nucleic acid synthesis? (RR) | back 104 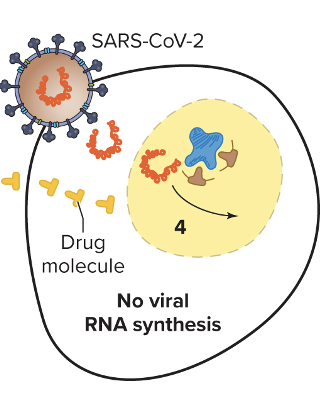 - Analogs are used, such as fake nucleotides, that mess with the DNA structure and inhibit proper binding of key molecules (prevent synthesis, etc.) 1. Remdesivir - purine analogs that terminate RNA replication in COVID 2. Ribavirin - purine analongs, used for hep C. |
front 105 Explain inhibition of nucleic acid synthesis without nucleotide analongs? | back 105 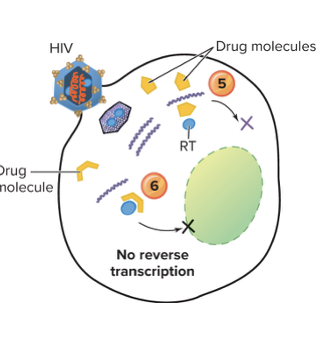 Non-nucleotide analogs are drugs that look similar to the natural building blocks (nucleotides) of DNA, but they aren’t real nucleotides.
|
front 106 Explain inhibition of viral assembly or release? | back 106 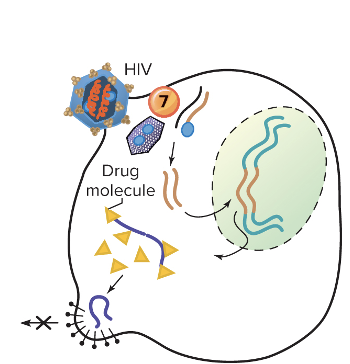 This prevents a mature virion from forming or being released. Ex. Indinavir and Saquinavir Protease inhibitors; insert into HIV protease, stopping its action and resulting in inactive noninfectious viruses -
|
front 107 What is antibiotic resistance? | back 107
|
front 108 What is the difference between intrinsic vs. acquired resistance? | back 108
_______________________________
|
front 109 What are the different ways to acquire antimicrobial resistance? | back 109 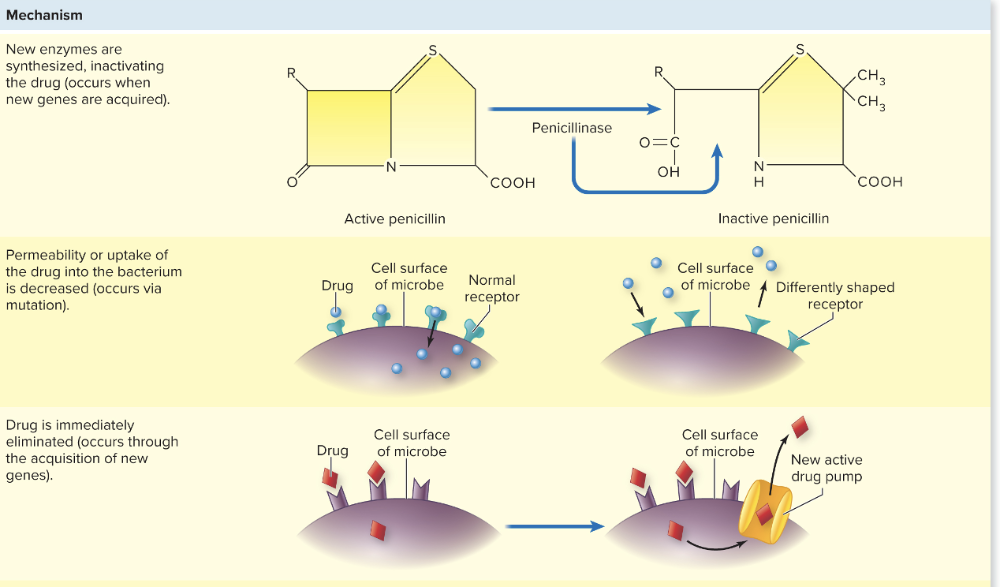 1. Mutation
2. Horizontal Transfer - GAIN new genes
3. New enzymes synthesized to inactivate the drug (occurs when new genes are acquired) 4. Drug is eliminated (through new genes which add pumps or transporters) 5. Binding sites can be decreased in number or affinity (due to new genes) 6. A new or alternative pathway the drug can act (due to mutations of original enzymes) |
front 110 True or False: Any large population of microbes is likely to contain a few individual cells that are naturally drug resistant | back 110 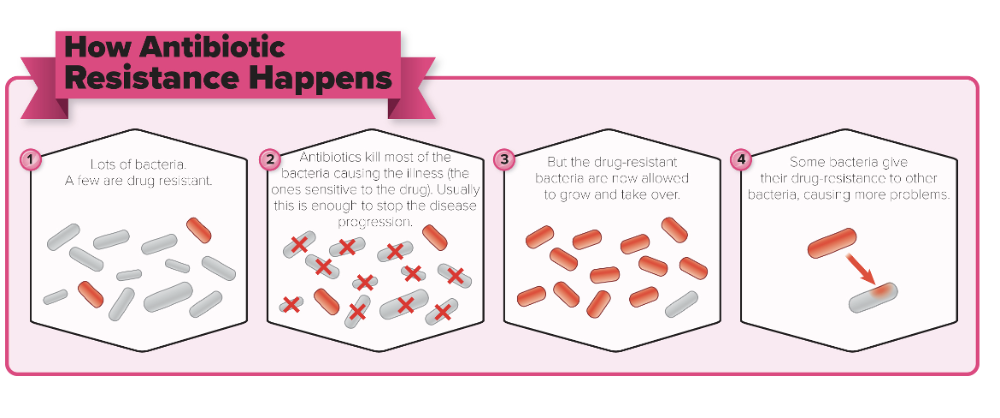 True
|
front 111 What are probiotics? | back 111 Preparations of live microorganisms fed to animals and humans to improve intestinal biota GIVES actual bacteria
Safe and effective |
front 112 What are prebiotics? | back 112
|
front 113 What's the main difference between probiotics and prebiotics | back 113
|
front 114 Chapter 20 | back 114 Infectious Diseases Manifesting in the Nervous System |
front 115 Describe the anatomy of the brain and spinal cord. | back 115 The brain and spinal cord is made up of neurons and surrounded by neurons. Brain is inside skull and spinal cord is inside the spinal column. Brain is surrounded by the meninges: (outside to inside) - Dura mater, arachnoid mater, and pia mater |
front 116 The CSF fills the _________, and is generally _____ in color. What are the two functions of the CSF? | back 116 Subarachnoid space clear, serum-like Provides liquid cushion for brain and spinal cord and provides nutrients to CNS |
front 117 What are the structural defenses of the nervous system? | back 117 - Bony casings of brain and spinal cord - CSF serves as cushion against impact - BBB (filter medications and microbes) |
front 118 What is the blood-brain barrier? | back 118 Cells that make up the walls of the blood vessels around the brain allow FEW molecules to pass (freer passage of ions, sugars, other metabolites) - PROHIBITS most microbes - HARD to introduce drugs and antibiotics to CNS - SELECTIVE |
front 119 True or False: There is NO immune privilege of the CNS. | back 119 False - CNS has a different or partial immune response when exposed to immunologic challenge - Functions in the CNS are vital for life, and the normal CNS can cause temporary damage - Cells in the CNS have lower levels of MHC antigens and complement proteins |
front 120 What two specialized cells in the CNS provide defenses to the body? | back 120 Microglial cells show phagocytic activity - Brain macrophages - Phagocytic activity is still LESS than other phagocytic cells in the body. |
front 121 True or False:
| back 121 FALSE |
front 122 True or False: There is no normal microbiota of the CNS and PNS. | back 122 True |
front 123 What is the gut-brain axis? | back 123 Recent microbiome studies reveal a relationship or potiental link between the gut microbiome and nervous system as gut microbiota may induce CNS autoimmunity and can cause changes in brain chemistry and behavior. |
front 124 What is meningitis? | back 124 Inflammation of the meninges
7 Different Types |
front 125 What can you do when you suspect someone has meningitis? | back 125 Obtain CSF through a lumbar puncture Gram stain and/or culture of CSF
Begin treatment with broad-spectrum antibiotics immediately (then shift treatment after confirmation of diagnosis) |
front 126 What are the typical symptoms of meningitis? (generally the same regardless of the cause) | back 126
|
front 127 True or False: In a healthy person, the blood-brain barrier and other defenses usually prevent microbes from entering the nervous system, so infections like meningitis are rare unless the barriers are compromised. | back 127 True
|
front 128 What is a conjugated vaccine? | back 128 Weak antigen (like a bacterial capsule made of sugar) is attached—or “conjugated”—to a strong protein antigen to make it more visible to the immune system.)
|
front 129 Which of the causative agents of meningitis have a vaccine for prevention? | back 129 Neiserria meningitdis, Streptococcus Pneumoniae, Haeomphilus Influenzae. |
front 130 Neisseria meningitidis causes what, and what information is relevant? | back 130 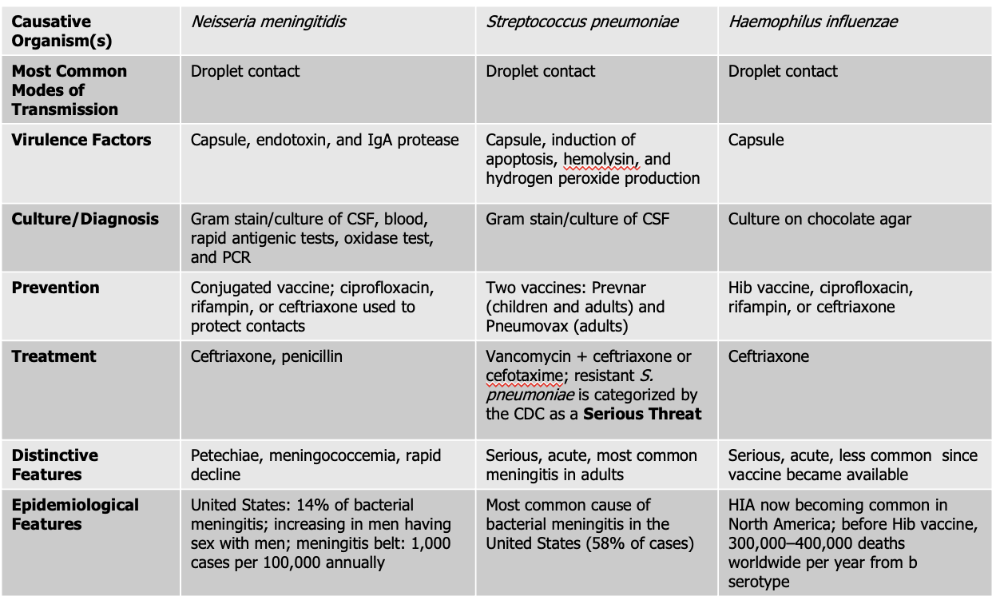
Mode of Transmission: DROPLET Virulence Factors:
Culture/Diagnosis: gram stain/culture of CSF, blood/rapid antigenic test, oxidase test, PCR Prevention: Conjugated vaccine, ciprofloxacin, rifampin, or ceftriaxone to protect contacts (CRC) Treatment: Ceftriaxone and Penicillin Distinctive features: petechiae, meningococcemia, rapid decline Epidemiological Features: 14% of bacterial meningitis; increasing in men having sex with men; meningitis belt: 1,000 cases per 100,000 annually |
front 131 How does neisseria meningitdis use the oxidase test? | back 131  Neisseria meningitidis is oxidase-positive, and the oxidase test helps confirm that. Here’s how it works:
|
front 132 What causative agent causes the most serious form for of acute meningitis? | back 132 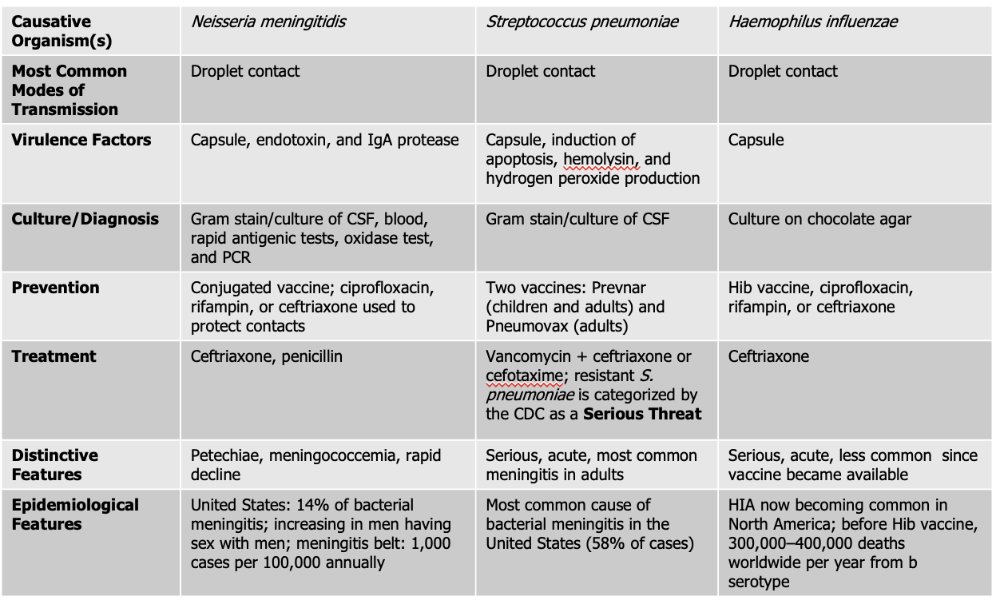 Neisseria Meningitdis *14% of all cases |
front 133 What causative agent is the most frequent cause of CAM (community-acquired meningitis) and bacterial meningitis? | back 133 Streptococcus pneumoniae. |
front 134 Streptococcus pneumoniae causes what, and what information is relevant? | back 134 
Mode of Transmission: DROPLET Virulence Factors:
Culture/Diagnosis: Gram stain/culture of CSF (distinct appearance on gram stain of CSF) Prevention: TWO vaccines Treatment: Vancomyocin + ceftriaxone or cefotaxime (resistant S. pneumoniae is serious threat) Distinctive features: Serious, acute, most common meningitis in adults Epidemiological Features: Most common cause of bacterial meningitis in the United States (58% of cases) |
front 135 What two products of S. pneumoniae further act as the virulence factors for meningitis? | back 135 Alpha hemolysis Hydrogen Peroxide |
front 136 Haemophilus influenzae causes what, and what information is relevant? | back 136 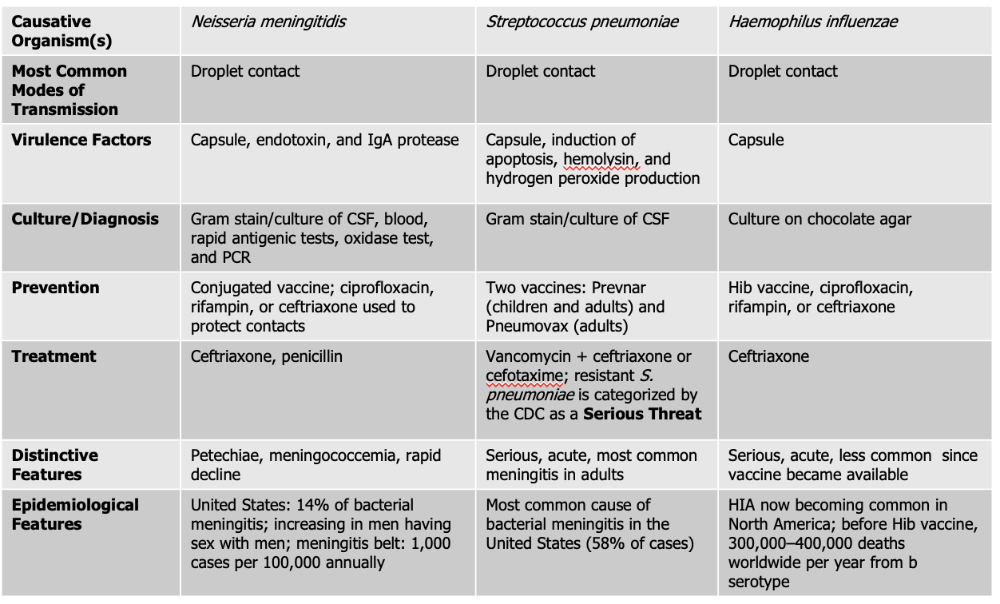
Mode of Transmission: DROPLET Virulence Factors: Capsule Culture/Diagnosis: culture on chocolate agar Prevention: HiB vaccine, ciprofloxacin, rifampin, ceftriaxone (CPC) Treatment: Ceftriaxone Distinctive features: Serious, acute, less common since vaccine was made Epidemiological Features: HIA now becoming common in North America; before Hib vaccine, 300,000–400,000 deaths worldwide per year from b serotype |
front 137 Which causative agent can cross the placenta and cause premature abortion and fetal death? | back 137 Listeria monocytogenes |
front 138 Listeria monocytogenes causes what, and what information is relevant? | back 138 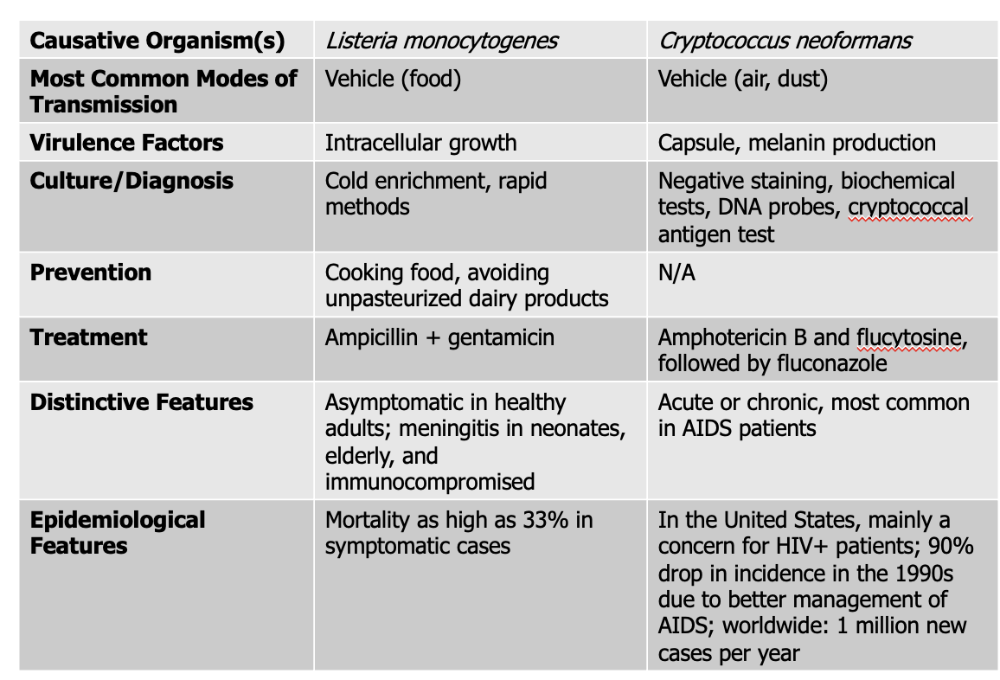
Mode of Transmission: Vehicle (Food) Virulence Factors: Intracellular growth Culture/Diagnosis: Cold enrichment, rapid methods Prevention: Cooking food, avoiding unpasteurized dairy products Treatment: Ampicillin + gentamicin Distinctive Features: asymptomatic in healthy people, meningitis in neonates, elderly, and immunocompromised Epidemiological Features: Mortality as high as 33% in symptomatic cases |
front 139 What is fastidious mean? | back 139 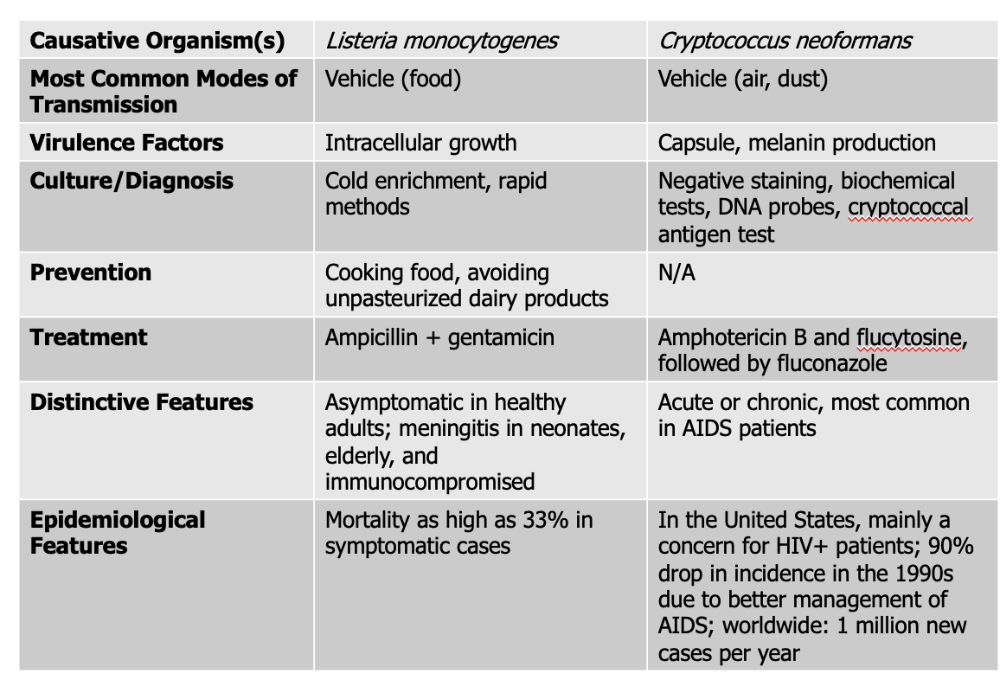 Fastidious means that a microorganism has very specific and complex nutritional or environmental requirements in order to grow. In other words, fastidious bacteria are “picky eaters” — they need special nutrients, conditions, or growth media that ordinary bacteria don’t. |
front 140 Cryptococcus neoformans causes what, and what information is relevant? | back 140 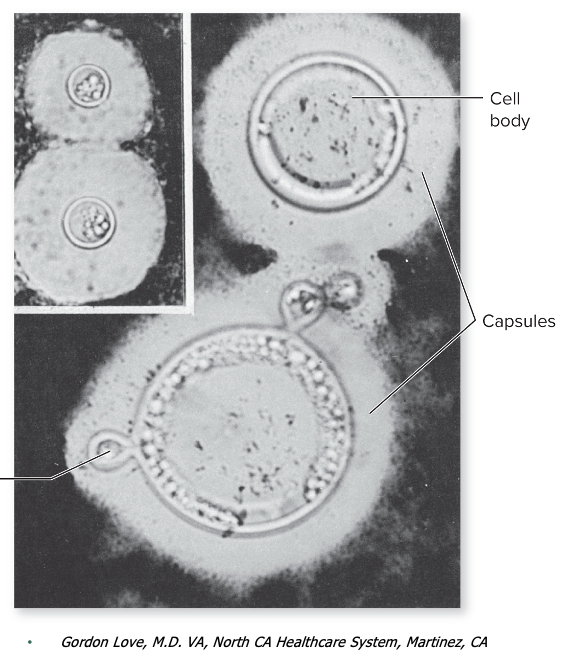
Mode of Transmission: vehicle (air, dust) Virulence Factors:
Culture/Diagnosis: Negative staining, biochemical tests, DNA probes, cryptococcal antigen test Prevention: None Treatment: Amphotericin B, flucytosine followed by fluconazole Distinctive Features: Acute or chronic, most common in AIDS patients Epidemiological Features: In the United States, mainly a concern for HIV+ patients; 90% drop in incidence in the 1990s due to better management of AIDS; worldwide: 1 million new cases per year |
front 141 Coccidiodes causes what, and what information is relevant? | back 141 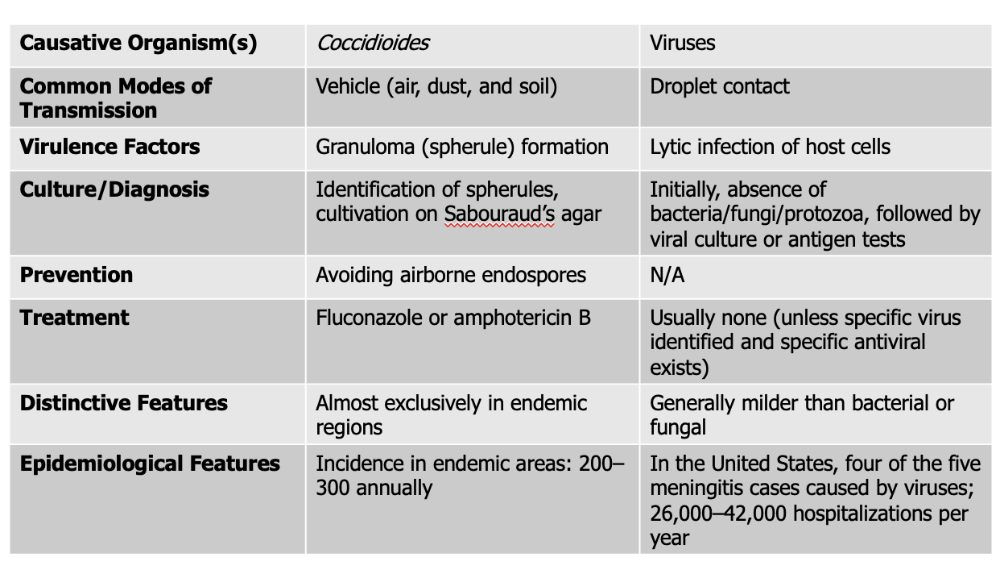
Mode of Transmission: Vehicle (air, dust, and soil) Virulence Factors: Granuloma (spherule) formation
Culture/Diagnosis: Identification of spherules, cultivation on Sabouraud’s agar Prevention: Avoiding airborne endospores Treatment: Fluconazole and Amphotericin B Distinctive Features: exclusively in endemic regions Epidemiological Features: Incidence in endemic areas: 200–300 annually |
front 142 Viral meningitis causes what, and what information is relevant? | back 142 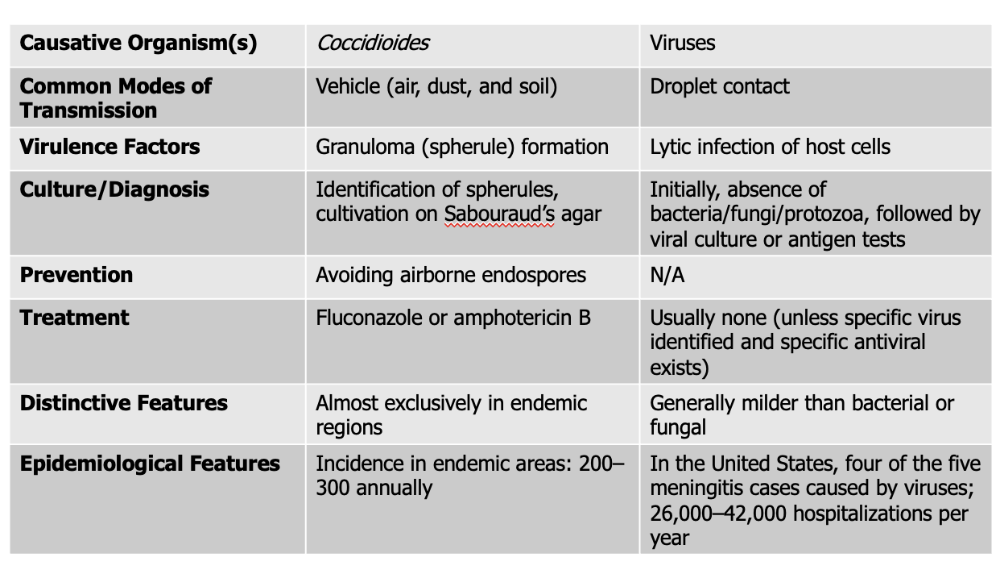
Mode of Transmission: DROPLET Virulence Factors: lytic infection of host cells Culture/Diagnosis: Initially, no microbe, followed by viral culture or antigen test Prevention: N/A Treatment: None (unless specific virus found and antiviral exists) Distinctive Features: milder than bacterial or fungal Epidemiological Features: In the United States, four of the five meningitis cases caused by viruses; 26,000–42,000 hospitalizations per year |
front 143 What are some basic facts about neonatal and infant meningitis? | back 143
|
front 144 What is the second most common cause of neonatal meningitis? | back 144 Escherichia coli (K1 strain) Mostly in premature babies |
front 145 What is the most common cause of neonatal meningitis? | back 145 Streptococcus agalactiae |
front 146 Streptococcus agalactiae causes what, and what information is relevant? | back 146 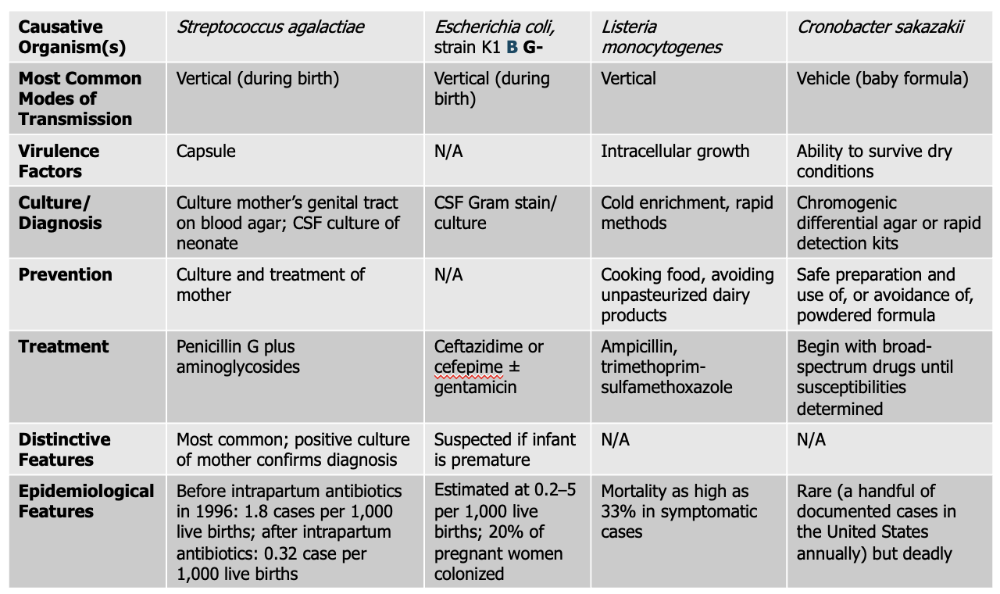
Mode of Transmission: Vertical (during birth) Virulence Factor: Capsule Culture/Diagnosis: Culture mother’s genital tract on blood agar; CSF culture of neonate Prevention: Culture and treatment of mother Treatment: Penicillin G plus aminoglycosides Distinctive Features: Most common, positive culture of mother confirms diagnosis Epidemiological Features: before intrapartum antibiotics in 1996: 1.8 cases per 1,000 live births; after intrapartum antibiotics: 0.32 case per 1,000 live births |
front 147 Escherichia coli causes what, and what information is relevant? | back 147
Mode of Transmission: Vertical (during birth) Virulence Factor: N/A Culture/Diagnosis: CSF Gram stain/culture Prevention: N/A Treatment: Ceftazidime or cefepime and/or gentamicin Distinctive Features: Suspected if infant is premature Epidemiological Features: Estimated at 0.2–5 per 1,000 live births; 20% of pregnant women colonized |
front 148 Listeria monocytogenes causes what, and what information is relevant? | back 148
Mode of Transmission: Vertical Virulence Factor: Intracellular growth Culture/Diagnosis: Cold enrichment, rapid methods Prevention: Cooking food, avoiding unpasteurized dairy products Treatment: Ampicillin, trimethoprim-sulfamethoxazole Distinctive Features: N/A Epidemiological Features: Mortality as high as 33% in symptomatic cases |
front 149 Cronobacter sakazakii causes what, and what information is relevant? | back 149
Mode of Transmission: Vehicle (baby formula) Virulence Factor: ability to survive dry conditions
Culture/Diagnosis: chromogenic differential agar or rapid detection kits Prevention: Self preparation, use of, or avoidance of powdered formula Treatment: broad-spectrum antibioitcs until main cause determined Distinctive Features: N/A Epidemiological Features: Rare (only handful of cases) but deadly |
front 150 What are the basic facts regarding meningoencephalitis? (MAN) | back 150 Inflammation of the brain (encephalitis) and meningitis - due to close association of the brain and spinal cord, infection of one can cause the other) Two causative agents are amoebas (protozoa): 1. Naegleria fowleri 2. Acanthamoeba |
front 151 _______ can enter the subarachnoid space causing ____________ (PAM)? | back 151 Naegleria fowleri; primary amoebic meningoencephalitis - causes rapid, destruction of brain and spinal tissue - Cases are rare, but the disease advances so rapidly that treatment is futile. |
front 152 Naegleria fowleri causes what, and what information is relevant? | back 152
Mode of Transmission: Vehicle (exposure while swimming in water) Virulence Factors: Invasiveness
Culture/Diagnosis: Examination of CSF, brain imaging, biopsy Prevention: limit warm freshwater or untreated tap water from entering nasal passages. Treatment: Pentamidine, Sulfadiazine Epidemiological Features: United States: zero to seven cases a year; 97% case fatality rate; spreading to northern states as climate warms |
front 153 _______ can cause ___________(GAM). | back 153 Acanthamoeba; granulomatous amoebic meningoencephalitis
|
front 154 Acanthamoeba causes what, and what information is relevant? | back 154
Mode of Transmission: Direct Contact Virulence Factors: Invasiveness Culture/Diagnosis: Examination of CSF, brain imaging, biopsy Prevention: N/A Treatment: Surgical excision of granulomas; pentamidine Epidemiological Features: Predominantly occurs in immunocompromised patients |
front 155 What are the characteristics of acute encephalitis? | back 155
|
front 156 What four pathogens cause acute encephalitis? (HAJI) | back 156 1. Arboviruses 2. HSV 1 or 2 3. JC virus 4. Post-infection encephalitis |
front 157 Arboviruses causes what, and what information is relevant? | back 157
Mode of Transmission: Vector (anthropod bites) Virulence Factors: (AFI)
Culture/Diagnosis: history, rapid serological tests, nucleic acids amplication Prevention: Insect Control Treatment: NONE Distinctive Features: history of exposure to insect is important |
front 158 HSV 1/2 causes what, and what information is relevant? | back 158
Mode of Transmission: Vertical or reactivation of latent infection Virulence Factors: N/A Culture/Diagnosis: Clinical presentation, PCR, Ab test, growth of virus in cell culture. Prevention: Maternal screening for HSV Treatment: Acyclovir Distinctive Features: In infants, disseminated disease present (rare between 30-50 years old) Epidemiological Features: HSV-1 is the most common cause of encephalitis (two cases per million per year) |
front 159 HSV 1/2 can cause _________ in what populations. | back 159 Acute encephalitis; newborns of HSV+ mothers Older children and young adults (5-30) Older Adults (+50) |
front 160 Which strain of HSV is most common in causing acute encephalitis? What happens that causes it? | back 160 HSV-1, represents a reactivation of dormant HSV from the trigeminal ganglion. |
front 161 What is the most common cause of acute encephalitis? | back 161 HSV-1 |
front 162 JV Virus causes what, and what information is relevant? | back 162
Mode of Transmission:
Virulence Factors: N/A Culture/Diagnosis: PCR of cerebrospinal fluid Prevention: None Treatment: No effective proven drug, but mefloquine has been used Distinctive Features: In SEVERELY immuncompromised (AIDS). Most people have this virus as normal microbiota, but when they become immunocompromised, they see the manifestation of this virus. Epidemiological Features: |
front 163 For what causative agent is the mode of transmission NOT from person to person — instead, it’s a complication that happens after a previous viral infection or vaccination. | back 163 Postinfection encephalitis - could also be due to overactivation of immune system |
front 164 Postinfection encephalitis causes what, and what information is relevant? | back 164
Mode of Transmission: sequelae of measles, other viral infections, occasionally vaccinations Virulence Factors: N/A Culture/Diagnosis: History of viral infection or vaccination Prevention: N/A Treatment: Steroids, anti-inflammatories Distinctive Features: Hist of virus/vaccine exposure critical Epidemiological Features: Rare in the United States due to vaccination; more common in developing countries, more common in boys than girls |
front 165 What are the three pathogens that cause subacute encephalitis? (PTS not the d unfortunely) | back 165 1. Toxoplasma gondii 2. Subacute sclerosing panencephalitis 3. Prions |
front 166 What causative agent for subacute encephalitis causes the "slow virus infection" | back 166 Subacute Sclerosing Panencephalitis (Measles Virus) |
front 167 What are the basic characteristics for subacute encephalitis? | back 167 - Symptoms take longer to show up, and they can be confused with other diagnosis
- Most common cause is Toxoplasma, and most common (postinfection viral) cause is measles. |
front 168 What is the difference between subacute and acute encephalitis? | back 168 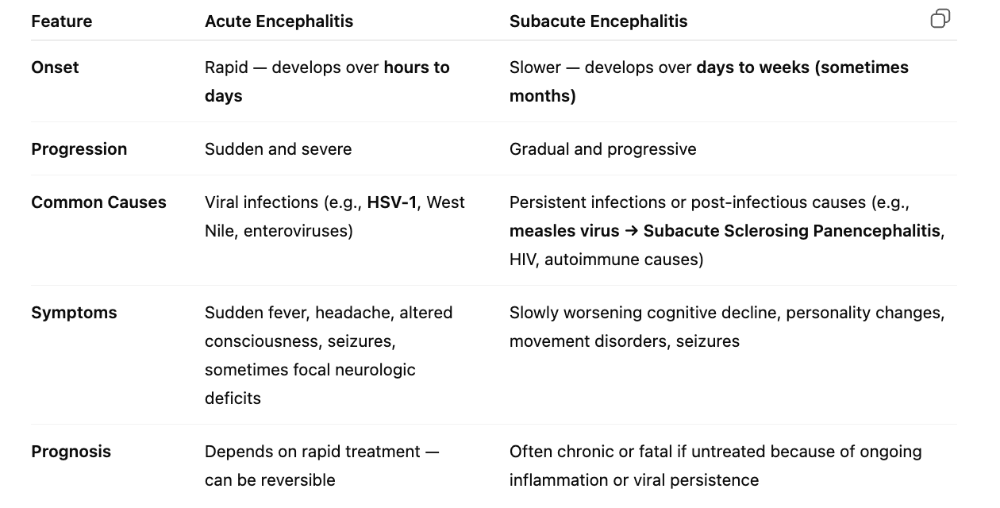 |
front 169 Toxoplasma gondi has two cell types, what are they, and what are they known for? | back 169 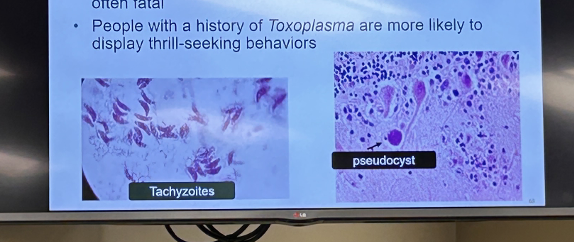 Tachyzoites and Pseduocysts |
front 170 Toxoplasma gondii causes what, and what information is relevant? | back 170
Mode of Transmission: Vehicle (meat) or fecal-oral Virulence Factors: intracellular growth Culture/Diagnosis: serological detection of IgM, culture, and histology Prevention: personal and food hygiene Treatment: pyrimethamine and/or leucovorin and/or sulfadiazine Distinctive Features: subacute, slower development of disease Epidemiological Features: considered a neglected parasitic infection (NPI)
|
front 171 What do IgM versus IgG antibodies show? | back 171 Here’s why:
|
front 172 What's the toxoplasma gondii life cycle? | back 172 Cats (the definitive hosts) release oocysts in their feces, which sporulate in the environment and can contaminate food or water. When intermediate hosts (like rats, mice, or humans) ingest these oocysts, the parasite travels through the bloodstream and can form cysts in tissues such as the brain and muscles. Infected rodents show altered behavior (less fear of predators), making them more likely to be eaten by cats — which continues the parasite’s life cycle. In humans, transplacental transmission can occur if a pregnant person becomes infected, potentially leading to congenital toxoplasmosis, which causes enlarged liver/spleen, hydrocephalus, or stillbirth. |
front 173 Toxoplasma gondii is very toxic for what human population? | back 173 - Pregnant People - 33% chance of mother transmitting infection to fetus via placenta Congential infection is in 1st or 2nd trimester - can lead to stillbirth, liver/spleen enlargement, liver failure, hydrocephalus, convulsions, damage to retina/blindness |
front 174 What is the primary reservoir (and what type of host) for toxoplasma gondii | back 174 Felines, both wild and domestic Cats serve as the reservoir and definitive host for Toxoplasma gondii because they’re the only animals in which the parasite can complete its sexual life cycle and shed infectious oocysts in their feces. The modes of transmission to humans are:
|
front 175 Subacute sclerosing panencephalitis (persistent measles infection) causes what, and what information is relevant? | back 175
Mode of Transmission: persistence of measles infection Virulence Factors: cell fusion, evasion of immune system Culture/Diagnosis: EEGs, MRI, serology (Ab versus measles virus) Prevention: None Treatment: None Distinctive Features: History of measles Epidemiological Features: Occurs in one in six people who have recovered from measles |
front 176 What are culture/diagnosis for the causative agent, SSP? (More info) | back 176 EEG
MRI
Serology / CSF antibody testing
|
front 177 True or False: Prions are infectious particles which hold nucleic acids. | back 177 False They are infectious cause they can cause disease but they have no genetic material |
front 178 How do prions cause disease? | back 178 In prion diseases, the normal brain protein (PrPᶜ) is misfolded into an abnormal, infectious form (PrPˢᶜ). This altered protein then induces other normal PrPᶜ proteins to also spontaneously misfold, creating a chain reaction. Over time, the accumulation of PrPˢᶜ proteins forms plaques and causes spongiform (hole-like) damage in the brain tissue, leading to severe neurological symptoms.
|
front 179 Prions causes what, and what information is relevant? | back 179
Mode of Transmission:
Virulence Factors: avoidance of host immune response
Culture/Diagnosis: Biopsy, image of brain Prevention: Avoiding infected meat or instruments; no prevention from inherited form Treatment: None Distinctive Features: Long incubation period; fast progression once it begins Epidemiological Features: CJD (one case per million worldwide, seen in older adults) and vCJD: 98% of cases in UK |
front 180 True or False: Zika virus is a DNA virus? | back 180 False; RNA virus |
front 181 What people are cautioned to not travel in Zika-endemic areas? Why? | back 181 Pregnant People
|
front 182 Zika virus causes what, and what information is relevant? | back 182
Mode of Transmission: Vertical (mother to placenta), vector-borne, sexual contact, possibly blood transfusions Virulence: proteins that reduces innate immune system response Culture/Diagnosis: PCR testing Prevention: avoiding mosquitos, NO VACCINE Treatment: supportive Epidemiological: Originated in Africa but spreading throughout world in 2016; small outbreaks in other parts of the world since |
front 183 What are the basic characteristics for rabies? What are the two types? (FD) | back 183 Slow, progressive zoonotic disease characterized by fatal encephalitis Virus enters the NS and causes involuntary control of muscles
|
front 184 What are some important aspects of Rabies Prevention and Treatment? | back 184 Treatment begins after EXPOSURE and BEFORE symptoms develop
|
front 185 Rabies viurs causes what, and what information is relevant? | back 185 
Mode of Transmission: Parenteral (bite trauma) and DROPLET Virulence: envelope glycoprotein
Culture/Diagnosis: DFA (direct fluorescent antibody test) Prevention: Inactivated vaccine Treatment: Postexposure passive and active immunization; induced coma and ventilator if symptoms start Epidemiological: United States: 1–5 cases per year; Worldwide: 35,000–55,000 cases annually |
front 186 What is poliomyelitis? | back 186
|
front 187 What are the three different diseases associated with Poliomyelitis? (PBP) | back 187
|
front 188 Poliomyelitis is neurotrophic, meaning it can infiltrates the ______ neurons of the ____________ | back 188 motor anterior horn of the spinal cord |
front 189 Polio viurs causes what, and what information is relevant? | back 189 
Mode of Transmission: fecal-oral, vehicle Virulence: attachment mechanisms Culture/Diagnosis: viral culture, serology Prevention: live attenuated vaccine (developing world) and inactivated oral vaccine (developed) Treatment: none, palliative, supportive Epidemiological: Polio has been 99% eradicated, except in Afghanistan and Pakistan, as of 2021 |
front 190 Which type of paralysis does botulism and tetanus respectively cause? They are both endo/exo toxin? | back 190 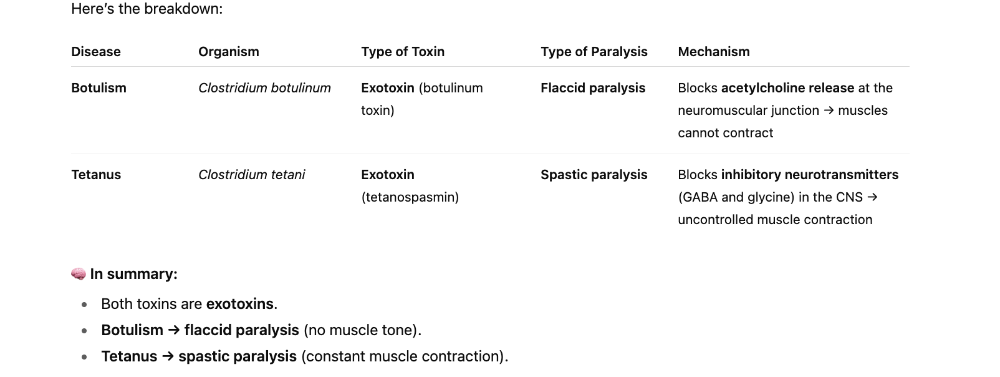 |
front 191 Clostridium tetani is what type of microbe? (ST) | back 191 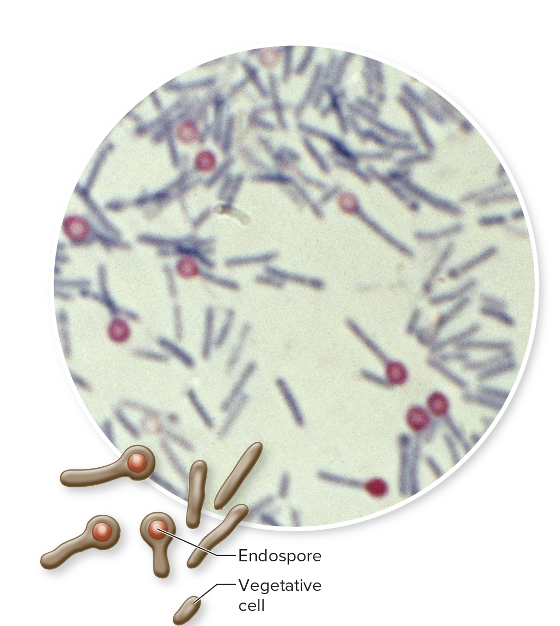 Gram+ Endospore Forming (under anaerobic conditions only) Rod |
front 192 Neonatal tetanus kills approximately ________ and the majority of infections are a direct result of ___________ | back 192 35,000 unhygienic practices during childbirth |
front 193 What is the biggest virulence factor for tetanus? | back 193
|
front 194 Clostridium tetani causes what, and what information is relevant? | back 194
Mode of Transmission: Parenteral, direct contact Virulence: tetanospasmin exotoxin Culture/Diagnosis: sympotamtic Prevention: Vaccination with tetanus toxoid (modified) in combination with diptheria and pertussis toxoid
Treatment: Combination of passive antitoxin and tetanus, toxoid active immunization, metronidazole and muscle relaxants, sedation Epidemiological: Worldwide, +/-25,000 newborn deaths annually in developing countries |
front 195 What are the basic characteristics of botulism? | back 195
|
front 196 What is the major virulence factor in botulism? | back 196 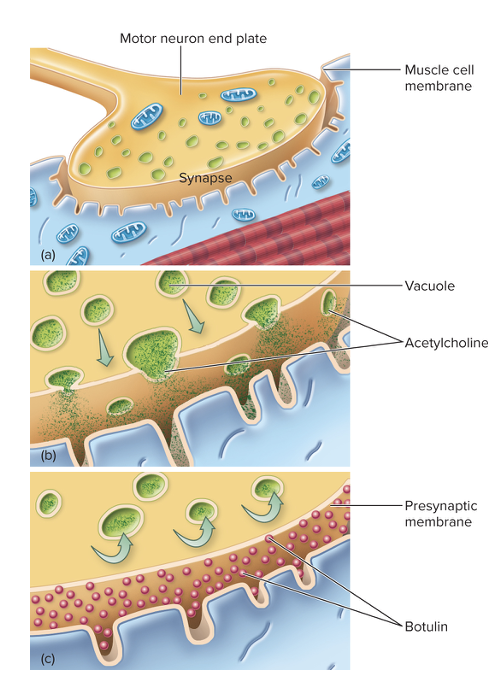
|
front 197 Clostridium botulinum causes what, and what information is relevant? | back 197
Mode of Transmission: Vehicle (foodborne toxin, airborne organism), direct contact (wound), and parenteral (injection) Virulence: botulinum exotoxin Culture/Diagnosis: culture of organism; demonstration of toxin Prevention: Food hygiene; toxoid immunization available for laboratory professional Treatment: Antitoxin, penicillin G for wound botulism, and supportive care Epidemiological: United States: 75% of botulism is infant botulism; over 150 cases annually Category A Bioterrorism Agent Epidemiological Features United States: 75% of botulism is infant botulism; over 150 cases annually Category A Bioterrorism Agent |
front 198 Trypanosoma brucei causes what, and what information is relevant? | back 198
Mode of Transmission: Vector, vertical Virulence: Immune evasion by antigen shifting
Culture/Diagnosis: microscopic examination of blood, CSF Prevention: vector control Treatment: Suramin or pentamidine (early), eflornithine or melarsoprol (late) Epidemiological: brucei gambiense: 7,000 to 10,000 cases reported annually; actual occurrence estimated at 600,000; T. brucei rhodesiense: estimated 30,000 cases occur annually |
front 199 For the nervous system, what are the two gram + endospore forming bacteria? | back 199 Clostridium tetani Clostridium botulinum |
front 200 For the nervous system, what are the three gram + bacteria? | back 200 Streptococcus pneumonia (meningitis) Listeria monocytogenes (meningitis, neonatal meningitis) Streptococcus agalactiae (neonatal meningitis) |
front 201 For the nervous system, what are the four gram - bacteria? | back 201
|
front 202 What are the two DNA viruses for the nervous system? | back 202 HSV 1/2 (encephalitis) JC virus (progressive multifocal leukoencephalopathy) |
front 203 What are the RNA viruses for the nervous system? | back 203 Arboviruses (encephalitis) Zika virus Poliovirus Measles virus (SSPE) Rabies virus |
front 204 What are the fungi in the nervous system? | back 204 Cryptococcus neoformans Coccidoides (Meningitis) |
front 205 What are the prions? | back 205 Creutzfeldt-Jacob prion (CJD) |
front 206 What are the protozoa (4) in the nervous system? | back 206 Naegleria fowleri (meningoencephalitis) Acanthamoeba (meningoencephalitis) Toxoplasma gondii (subacute encephalitis) Trypanosoma brucei (ASS) |
front 207 Chapter 22 | back 207 Infectious Diseases Manifesting in the Respiratory System |
front 208 Where does the lower respiratory tract begin? | back 208 Trachea, then into the bronchi, bronchioles, and alveoli |
front 209 What are the anatomical defenses of the respiratory tract? | back 209
|
front 210 What are the additional defenses of the respiratory system? | back 210
|
front 211 What is the normal microbiota in the respiratory tract? | back 211 - large number of commensal organisms (one species gains benefits) due to constant contact with environment
|
front 212 Infectious Disease of the Upper Respiratory Tract | back 212
|
front 213 Over 200 different types of viruses causes what, and what information is relevant? | back 213
Mode of Transmission: droplets and indirect Virulence: attachment proteins, most symptoms induced by host response
Culture Diagnosis: Not needed Prevention: No vaccine (HYGIENE) Treatment: ONLY supportive care no chemotherapeutic agents Epidemiology: highest incidence among preschool and elementary schoolchildren, with average of three to eight colds per year; adults and adolescents: two to four colds per year |
front 214 What are the basic characteristics to a sinusitis? | back 214
|
front 215 Viruses causes what, and what information is relevant? | back 215
Mode of Transmission: direct and indirect contact Culture Diagnosis: culture not performed, diagnoses based on clinical presentation Prevention: hygiene Treatment: none Distinctive features: viral and bacteria more common Epidemiology: follows common cold |
front 216 Various bacteria (mixed infection) causes what, and what information is relevant? | back 216
Mode of Transmission: Endogenous (opportunism) Culture Diagnosis: culture not performed, diagnoses based on clinical presentation; occasionally X-rays or imaging used Prevention: N/A Treatment: no antibiotic unless unresolved for some weeks Distinctive Features: viral and bacterial more common than fungal Epidemiology: United States: affects 1 of 7 adults; between 12 and 30 million diagnoses per year |
front 217 Various fungi causes what, and what information is relevant? | back 217
Mode of Transmission: introduction by trauma or opportunistic growth Culture Diagnosis: culture not performed, diagnoses based on clinical presentation; occasionally X-rays or imaging used Prevention: N/A Treatment: Physical removal of fungus, or anti-fungals in severe cases Epidemiology: fungal sinusitis varies with geography; in the United States: more common in SE and SW; internationally: more common in India, North Africa, Middle East |
front 218 What are common characteristics to acute otitis media? | back 218 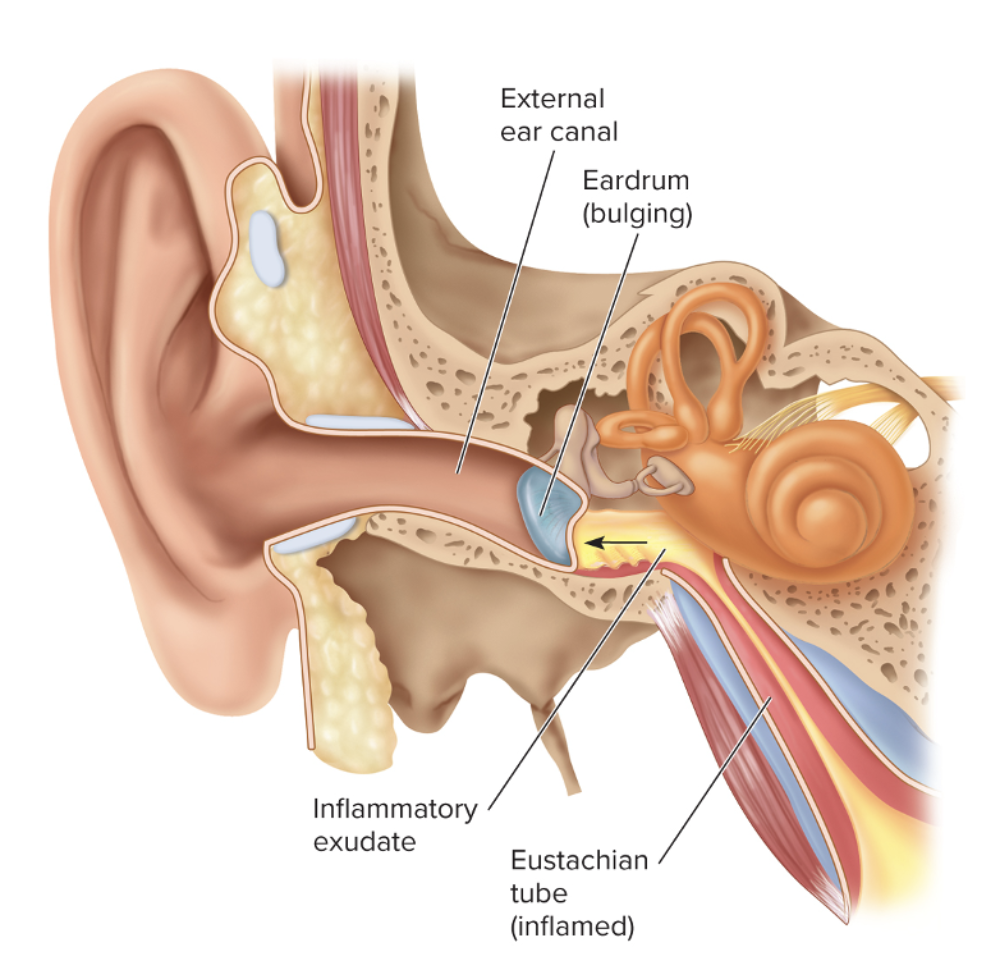
|
front 219 What generally causes chronic otitis media? | back 219 Fluids builds up in eustachian tubes
|
front 220 What is the secreted fluid called? | back 220 Effusion |
front 221 Streptococcus pneumoniae causes what, and what information is relevant? | back 221
Mode of Transmission: endogenous *may follow upper respiratory tract infection by S. pneumoniae) Virulence: Capsule OR hemolysin
Culture/Diagnosis: clinical symptoms and failure to resolve in 72 hours Prevention: Pneumococcal conjugate vaccine (PCV13) Treatment:
Epidemiological: 30% of cases in the U.S |
front 222 Candida auris causes what, and what information is relevant? | back 222
Mode of Transmission: not KNOWN Virulence: biofilm formation Culture/Diagnosis: MALDI-TOF or PCR; CDC will identify if requested Prevention: None Treatment: Consult w/ CDC (antibiotic resistance) Epidemiological: First appeared in 2009; increasing in U.S. |
front 223 What are the common characteristics of pharyngitis? (compare viral and bacteria) | back 223
|
front 224 What are the three causative agents of pharyngitis? (SFV, not SFG) | back 224
|
front 225 What is a major causative agent of pharyngitis? | back 225 Streptococcus pyogenes
Virulence
|
front 226 Streptococcus pyogenes causes what, and what information is relevant? | back 226
Mode of Transmission: Droplet or Direct Virulence:
Culture/Diagnosis: beta-hemolytic on blood agar, sensitive to bacitracin Prevention: hygiene Treatment: penicillin, cephalexin (allergic to penicillin) Distinctive Features: more severe than viral Epidemological: United States: 10% to 20% of all cases of pharyngitis |
front 227 Fusobacterium necrophorum causes what, and what information is relevant? | back 227
Mode of Transmission: endogenous Virulence: Invasiveness, endotoxin Culture/Diagnosis: Culture anerobically, CT for abscess Treatment: Penicillin Distinctive: can lead to Lemierre's syndrome Epidemiological: Causes up to 15% of acute pharyngitis in teens/young adults |
front 228 Viruses causes what, and what information is relevant? | back 228
Mode of Transmission: all forms of contact Culture/Diagnosis: Goal is to rule out S. pyogenes (and F. necrophorum); further diagnosis usually not performed Prevention: Hygiene Treatment: Symptom relief Distinctive: hoarseness Epidemological: ubiquitous; responsible for 40% to 60% of all pharyngitis |
front 229 Diseases in Both Upper and Lower Respiratory Tracts | back 229 ONLY 3 - Whooping Cough, RSV, and Influenza |
front 230 What is the basic information regarding whooping cough, and what are the three stages? (CPC) | back 230
|
front 231 T or F: Bordetella pertussis is gram + and anerobic | back 231 False; small, gram - STRICTLY aerobic |
front 232 Bordetella pertussis causes what, and what information is relevant? | back 232
Mode of Transmission: DROPLET contact Virulence:
Culture/Diagnosis: PCR or growth on blood agar, charcoal, or potato-glycerol agar, diagnosis can be made on symptoms Prevention: high vaccination coverage has kept incidence low in the U.S. Current vaccines are acellular formation of antigens. Booster needed after 11 y/o Treatment: Azithryomycin; drug resistant B. pertussis is concerning threat Epidemiological: United States: 19,000 cases in 2017, 14,000 in 2018; internationally: hundreds of millions of cases annually |
front 233 What are the basic characteristics for RSV? | back 233
|
front 234 Respiratory syncytial virus causes what, and what information is relevant? | back 234
Mode of Transmission: droplet and indirect Virulence: syncytia formation Culture/Diagnosis: RT-PCR Prevention: Passive-antibody (humanized monoclonal) in high-risk children Treatment: ribavirin plus passive antibody for severe cases Epidemiological: United States: general population, less than 1% mortality rates, 3% to 5% mortality in premature infants or those with congenital heart defects; internationally: seven times higher fatality rate in children in developing countries |
front 235 What are the basic characteristics for Influenza | back 235
|
front 236 What are the influenza glycoproteins? | back 236 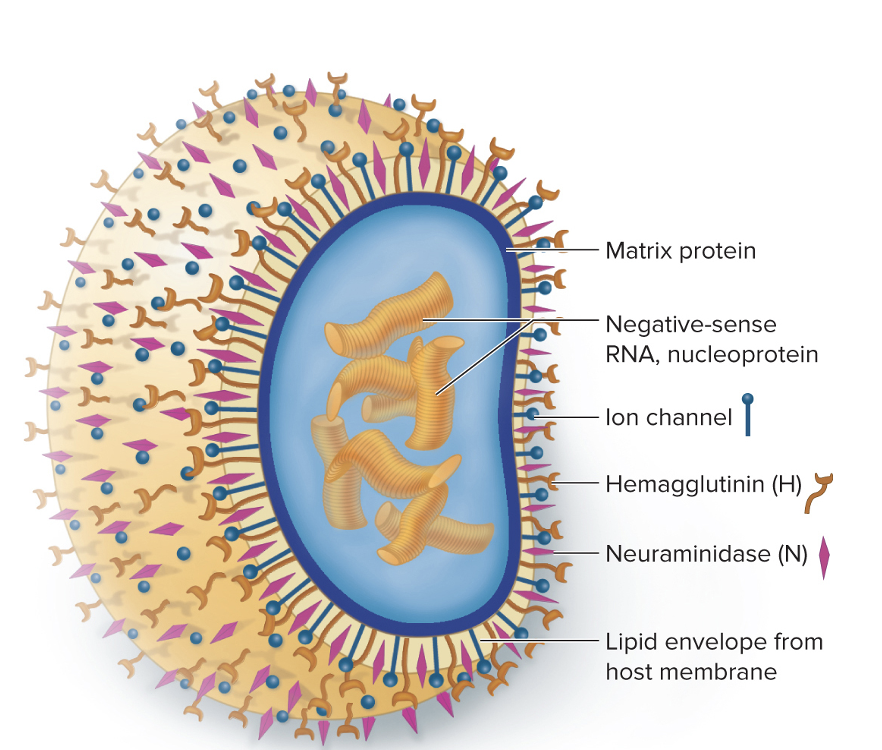 Hemagglutinin (H):
Neuraminidase (N):
|
front 237 What is the difference between antigenic drift and antigenic shift? | back 237 We get flu shots every year because the glycoproteins (hemagglutinin and neuraminidase) on the influenza virus mutate frequently — a process called antigenic drift.
|
front 238 The _______ air of __________ facilitates the spread of the virus as it helps the virus remains airborne for more extended periods of time | back 238 drier winter
|
front 239 Influenza A, B, and C viruses causes what, and what information is relevant? | back 239
Mode of Transmission: Droplet, direct, and indirect Virulence: Glycoprotein spikes, antigenic shift and drift Culture/Diagnosis: RT-PCR Prevention: vaccines
Treatment: Oseltamivir (Tamiflu), baloxavir (Xofluza) Epidemiological: For seasonal flu, deaths vary from year to year; United States: range from 17,000 to 52,000; internationally: range from 250,000 to 500,000 |
front 240 What are the four infectious diseases only in the lower respiratory system? | back 240 1. Tuberculosis 2. Community-acquired pneumonia (3 causes) 3. Hospital-acquired pneumonia 4. Hantavirus pulmonary syndrome |
front 241 What are the basic characteristics about Tuberculosis? | back 241
|
front 242 What are the different types of tuberculosis? | back 242  1. Primary
2. Extrapulmonary TB
3. Secondary (reactivation) TB
|
front 243 What are the different testing methods for TB? (5) | back 243 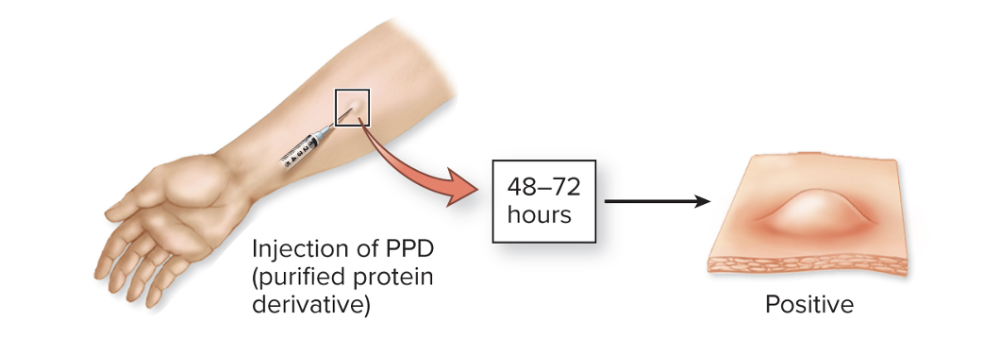 1. The TB skin test (Mantoux test)
Here’s what happens:
So the welt doesn’t mean you currently have active TB — it just means your immune system has seen TB antigens before (from infection or vaccination). 2. IGRA: blood test to determine T-cell reactivity to M. tuberculosis IGRA stands for Interferon-Gamma Release Assay — it’s a blood test used to detect Mycobacterium tuberculosis infection (like the TB skin test, but more specific). Here’s how it works |
front 244 Mycobacterium tuberculosis causes what, and what information is relevant? | back 244
Mode of Transmission: Vehicle (airborne) Virulence: Lipids in wall, ability to stimulate strong cell-mediated immunity (CMI) Culture/Diagnosis: Culture, PCR test (Xpert®), IGRA, complemented by skin test and chest X-ray Prevention: Avoiding airborne M. tuberculosis AND BCG vaccine in other countries Treatment:
Distinctive Features: Remains airborne for long periods; extremely slow-growing, which has implications for diagnosis and treatment Epidemiological: remains airborne for long periods; extremely slow-growing, which has implications for diagnosis and treatment |
front 245 MDR-TB and EDR-TB causes what, and what information is relevant? | back 245
Mode of Transmission: Vehicle (airborne) Virulence: lipids in wall, ability to stimulate strong cell-mediated immunity (CMI) Culture/Diagnosis: culture, PCR test (Xpert®), IGRA, complemented by skin test and chest X-ray Prevention: avoiding airborne M. tuberculosis; BCG vaccine in other countries Treatment: multiple-drug regimen, which may include pretomanid, bedaquiline; and linezolid; in Serious Threat category in CDC Antibiotic Resistance Report Distinctive Features: much higher fatality rate over shorter duration Epidemiological: United States: a fewer cases per year; worldwide: 500,000 new infections with MDR-TB in 2020 |
front 246 What is the basic characteristics of pneumonia? | back 246
|
front 247 What are the 6 different causative agents for CAP, separated based on mode of transmission? RSM HPL | back 247 1. Droplet
2. Vehicle (Inhalation of Spores)
3. Vehicle (Water Droplets)
|
front 248 Rhinoviruses causes what, and what information is relevant? | back 248 Mode of Transmission: Droplet contact (or endogenous transfer) Virulence: N/A Culture/Diagnosis: Failure to find bacteria or fungi Prevention: Hygiene Treatment: None Distinctive Features: Mild Epidemiological: 9% of CAP cases |
front 249 Streptococcus pneumonia causes what, and what information is relevant? | back 249 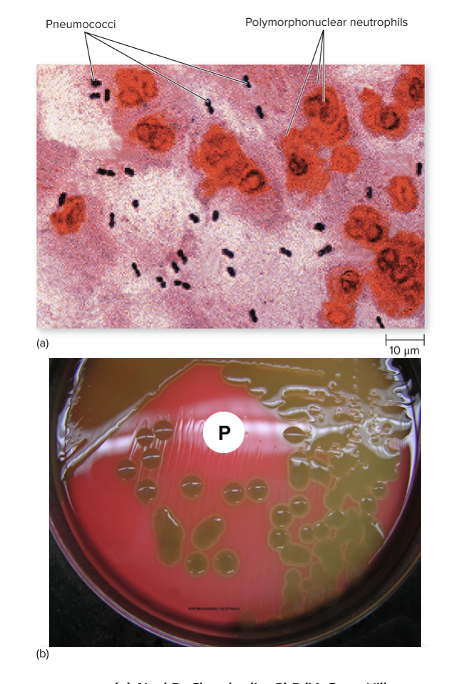
Mode of Transmission: Droplet contact (or endogenous transfer) Virulence: CAPSULE
Culture/Diagnosis: Gram-stain, alpha-hemolytic on blood agar Prevention: Vaccine (children and older adults) - PCV13 or PPSV23 Treatment: doxycycline, ceftriaxone, with or without vancomycin; much resistance Distinctive Features: SEVERLY ILL
Epidemiological: 5% of cases, serious threat |
front 250 Mycoplasma pneumoniae causes what, and what information is relevant? | back 250
Mode of Transmission: Droplet Virulence: Adhesins Culture/Diagnosis: Rule out other etiologic agents; serology; PCR Prevention: No vaccine, no permanent immunity Treatment: erythryomycin Distinctive Features: mild, walking pneumonia |
front 251 Histoplasma capsulatum causes what, and what information is relevant? | back 251
Mode of Transmission: Vehicle (inhalation of spores in contamined soil) Virulence: Survival in phagocytes Culture/Diagnosis: Rapid antigen tests, microscopy Prevention: Avoid soil with bird and bat droppings Treatment: Itraconazole Distinctive Features: many asymptomatic infections Epidemiological: the United States, 250,000 infected per year; 5% to 10% have symptoms |
front 252 Pneumocystitis jirovecii causes what, and what information is relevant? | back 252
Mode of Transmission: Vehicle (inhalation of spores) Virulence: N/A Culture/Diagnosis: Microscopy Prevention: Antibiotics given to AIDS patients Treatment: Trimethoprim/sulfamethoxazole Distinctive Features: mostly occurs in AIDS patients Epidemiological: exclusively in severely immunocompromised patients |
front 253 Legionella pneumophila causes what, and what information is relevant? | back 253
Mode of Transmission: Vehicle (water droplets) Virulence: N/A Culture/Diagnosis: Urine antigen test, culture requres selective charcoal yeast extract agar Prevention: N/A Treatment: Fluoroquinolone, azithromycin, clarithromycin Distinctive Features: Mild pneumonias in healthy people; can be severe in elderly or immunocompromised Epidemiological: United States: on the rise; 6,000 to 8,000 cases annually |
front 254 Gram-negative and gram-positive bacteria from upper respiratory tract or stomach; environmental contamination of ventilator causes what, and what information is relevant? | back 254
Mode of Transmission: Endogenous (aspiration) Virulence: N/A Culture/Diagnosis: culture of lung fluids Prevention: Elevating patient’s head, preoperative education, care of respiratory equipment Treatment: Varies by etiology Epidemiological: United States: 300,000 cases per year; occurs in 0.5% to 1.0% of admitted patients; mortality rate in the United States and internationally is 20% to 50% |
front 255 What are the basic characteristics for Hantavirus? | back 255
|
front 256 Hantavirus causes what, and what information is relevant? | back 256 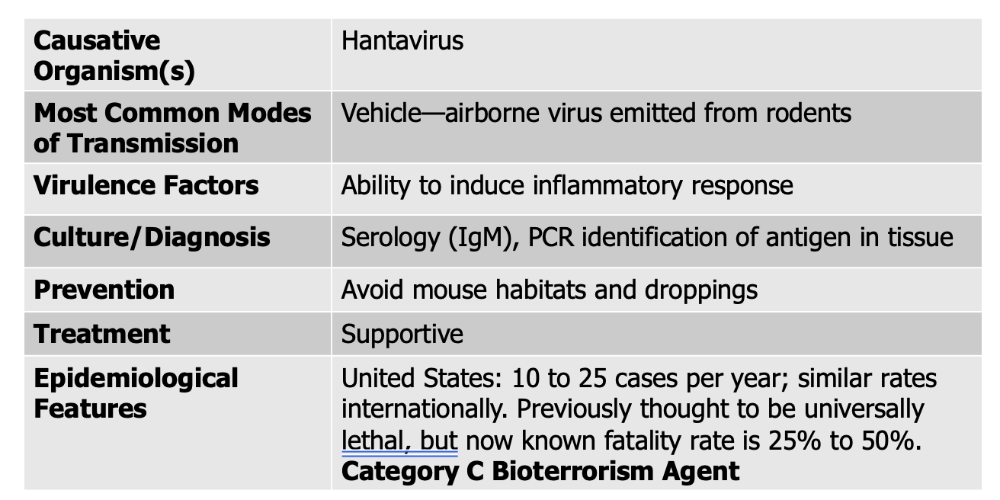 ** virulence factor here is not the virus itself, but it causes overactivation of the immune system leading to respiratory distress and inflammatory response Transmission and epidemiology:
Treatment and prevention:
|