Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
lec 12-15
front 1 mutation | back 1 heritable change in DNA sequence ( permanent, passed down) that can lead to a change in phenotype (observable properties of an organism) |
front 2 mutant (the organism that has that chain) | back 2 a strain of any cell or virus differing from parental strain (wildtype strain) in genotype (nucleotide sequence of genome) |
front 3 wild-type strain | back 3 typically refers to strain isolated from nature (from the parent) |
front 4 selectable mutations | back 4 those that give the mutant a growth advantage under certain conditions or a distinguishable phenotype - like antibiotic resistance useful in genetic research |
front 5 nonselectable mutations | back 5 those that usually have neither an advantage nor a disadvantage over the parent - maybe changed the metabolic pathway slightly but the cell growth just fine detecting such mutations requires examining a large # of colonies and looking fr differences (screening) |
front 6 screening basically means ___ | back 6 hard process where you have to examine thousands of colonies individually to find the one with the subtle change you're looking for its tedious compared to the selection |
front 7 one classic screening methods involve ____ | back 7 finding nutritional auxotroph using negative selections and replica plating |
front 8 replica plating is ___ | back 8 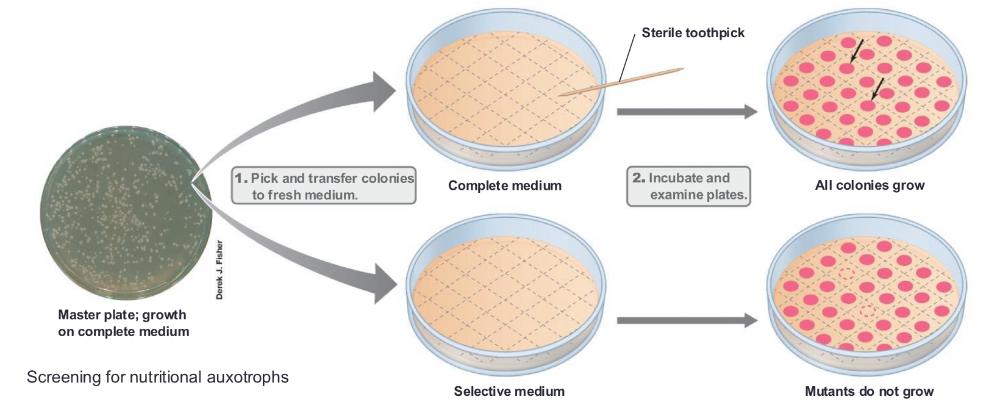 one of the methods available to facilitate screening it is useful for identifying cells with a nutritional requirement for growth (auxotroph) |
front 9 auxotroph is ___ | back 9 a mutant that has lost its ability to make some essential nutrients so it now requires that nutrient in its growth medium so it needs help. |
front 10 screening for nutritional auxotrophs process: | back 10 so the process starts w a master plate:
|
front 11 induced mutations | back 11 those made environmentally or deliberately can result from exposure to natural radiation or oxygen radicals |
front 12 spontaneous mutations | back 12 those that occur without external intervention (usually mistake during DNA replication, or DNA damage, too many radicals, etc) |
front 13 point mutations | back 13 mutations that change only one base pair (one nucleotide in genome that changed) can lead to single amino acid change in a protein, an incomplete protein, or no change at all |
front 14 cells dont have any ____ | back 14 resistance |
front 15 reason for mutation? | back 15 antibiotic is a selection pressure, mutation is random |
front 16 what factor promotes mutation? | back 16 mutagen, chemicals, and viruses that damage DNA |
front 17 silent mutation | back 17 does not affect amino acid sequence (nucleotide sequence change but no amino acid changes) |
front 18 missense mutation | back 18 amino acid changed; polypeptide altered (nucleotide sequence change and amino acid changes) |
front 19 nonsense mutation | back 19 codon becomes stop codon; polypeptide is incomplete (coding codon->stop codon) |
front 20 types of mutation (photo) | back 20 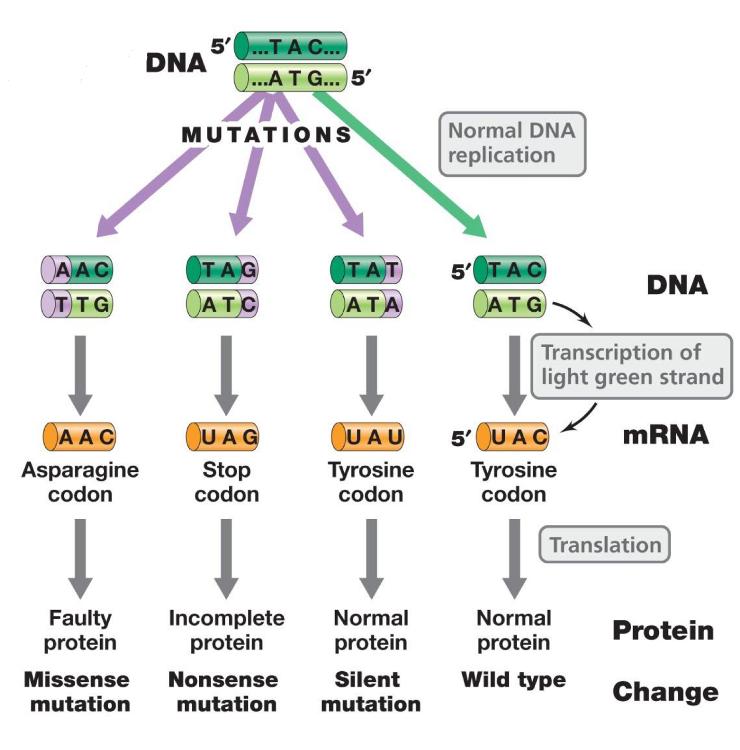 |
front 21 deletions and insertions cause more ____ | back 21 dramatic changes in DNA (frameshift mutation) |
front 22 frameshift mutations | back 22 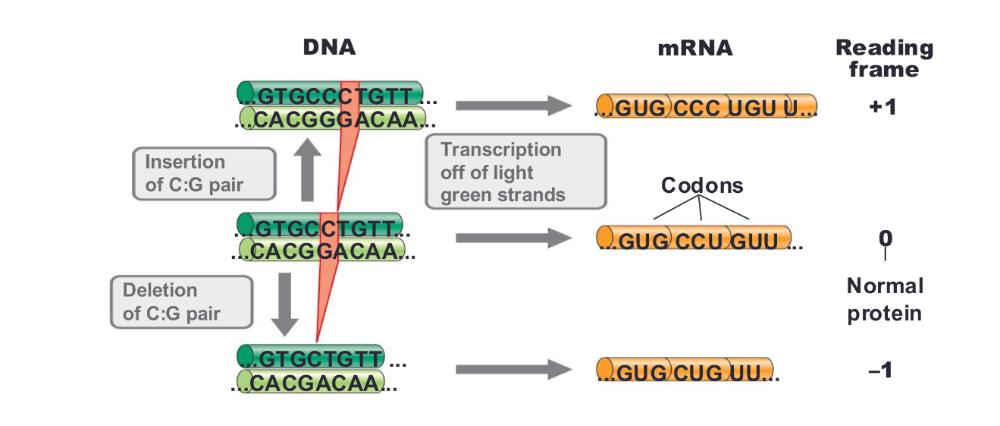 deletions or insertions that result in a shift in the reading frame often result in complete loss of gene function |
front 23 point mustions are typically ___ | back 23 reversible |
front 24 reversion | back 24 alteration in DNA that reverses the effects of a prior mutation (second mutation corrected the first mutation) |
front 25 revertant | back 25 strain in which original phenotype is restored (there are two types) |
front 26 a type of revertant: same site revertant | back 26 mutation is at the same site as original mutation |
front 27 a type of revertant: second-site revertant (the same as suppressor) | back 27 mutation is at a different site in the DNA |
front 28 suppressor | back 28 mutation that compensates for the effect of the original mutation |
front 29 reversion and suppressor (photo) | back 29 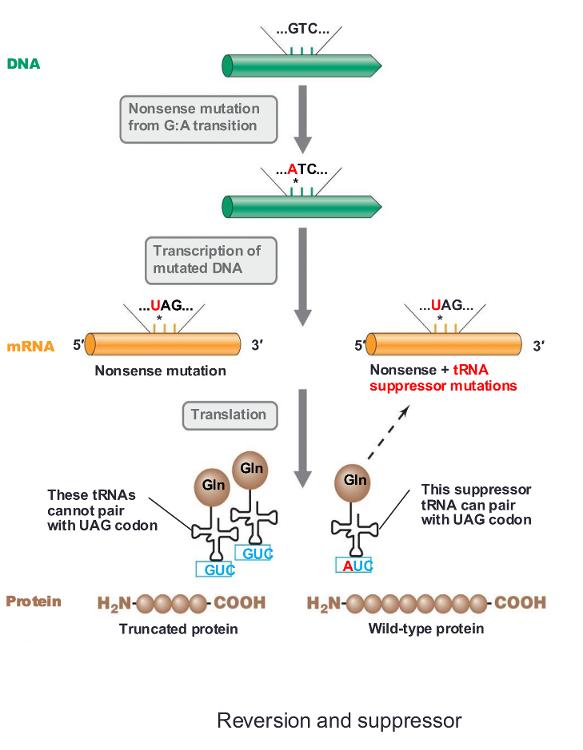 |
front 30 for most microorganisms, errors in DNA replication occur at a frequency of ____ | back 30 10^-6 to 10^7 /kb (per gene) |
front 31 the Ames test makes ____ | back 31 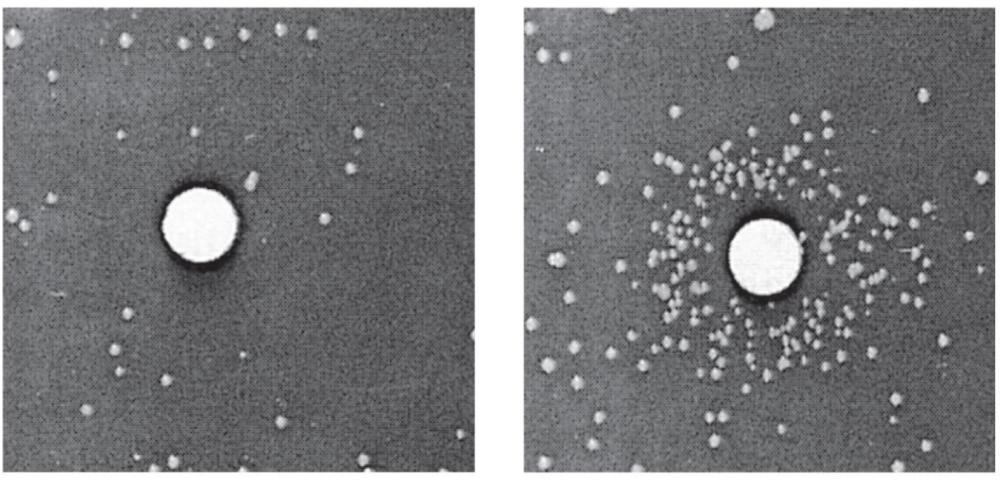 practical use of bacterial mutations to detect for potentially hazarduous chemicals |
front 32 the Ames test looks for an increase in ___ | back 32 mutations of bacteria in the presence of suspected mutagen - a wide variety of chemicals have been screened for toxicity and carcinogenicity |
front 33 mutagens | back 33 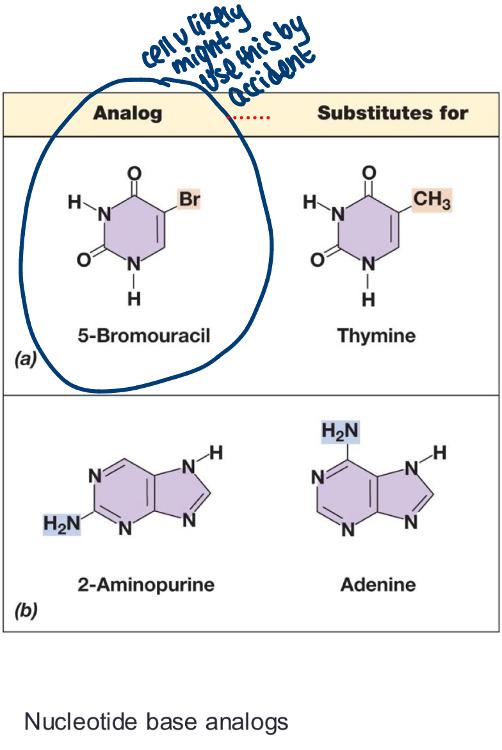 chemical, physical, or biological agents that increase mutation rates (several classes of chemical mutagens exist) |
front 34 a class of chemical mutagens: nucleotide base analogs | back 34 resemble nucleotides, directly incorporated into DNA, introduce mutatiosn during replication they look like nucleotide, but they are not |
front 35 chemical mutagens that induce ____ | back 35 chemical modifications, for example, alkylating agents such as nitrosoguanidine already in DNA, not thru the bases but react directly thru DNA bases -> altering structure |
front 36 a class of chemical mutagens: intercalating agents | back 36 that insert between base pairs and cause frameshift mutations - for example, ethidium bromide deletions or insertions some chemical, flat, can insert between DNA replication |
front 37 two main categories of mutagenic electromagnetic radiations: | back 37 non-ionizing and ionizing |
front 38 non-ionizing (i.e., UV radiation) | back 38
|
front 39 ionizing (i.e., x-rays, cosmic rays, and gamma rays) | back 39
|
front 40 there ___ types of DNA repair systems | back 40 3 |
front 41 direct reversal (type of DNA repair system) | back 41 mutated base is still recognizable and can be repaired without referring to other strand (ligase) enzyme can undo the damage restoring the residue base without needing to cut the DNA backbone or use the template |
front 42 repair of single-strand damage (type of DNA repair system) | back 42 damaged DNA is removed and repaired of using opposite strand as template basic incision repair nucleotide incision repair |
front 43 repair of double-strand damage (type of DNA repair system) | back 43 a break in the DNA can lead to chromosome fragmentation
|
front 44 perfect fidelity in organisms is counterproductive because ____ | back 44 it prevent evolution |
front 45 the mutation rate of an organism is subject to ___ | back 45 change |
front 46 mutant can be isolated from strains that are ____ | back 46 hyperaccurate or hypermutable |
front 47 mutator (hypermutable) strains: | back 47 bacteria that benefit from increased mutation rates, such as dnaQ mutants (dnaQ encodes the proof-reading enzyme in DNA polymerase III) - the enzyme that copies DNA, if dnaQ is mutated, the dna polymerase makes. more mistakes and mutation rate of the cell increases |
front 48 SOS regulatory system ___ | back 48 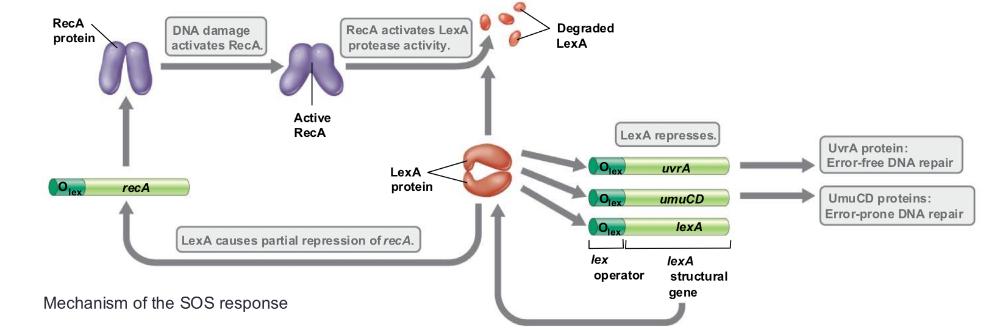 is more error-prone. it allows replication to proceed and cell to replicate, but errors are more likely carried out by DNA polymerases IV and V SOS system allows DNA to be synthesized with no template when DNA damage is large scale, the cell may use a different type of repair system (i.e., damage interferes with DNA replication) image: 1. RecA gets activates and then acts like a coprotease 2. which activates lexA to cleave itself (to be destroyed), its a repressor protein, that normally sits on the DNA and blocks the expression of genes of DNA repair (gets degraded) 3. break comes off, the gene that it was repressing get switched on when lexA levels drop: (they form component of polymerase) they are low fidelity transleisiancy polymerases- just copy pass damaged dna polymerases, they guess or skip over the DNA damaged part uvrA partial repression (part of more accurate nucleotide excision repair pathway) umuCD full activation of genes trading accuracy for survival |
front 49 three mechanisms of genetic exchange: | back 49 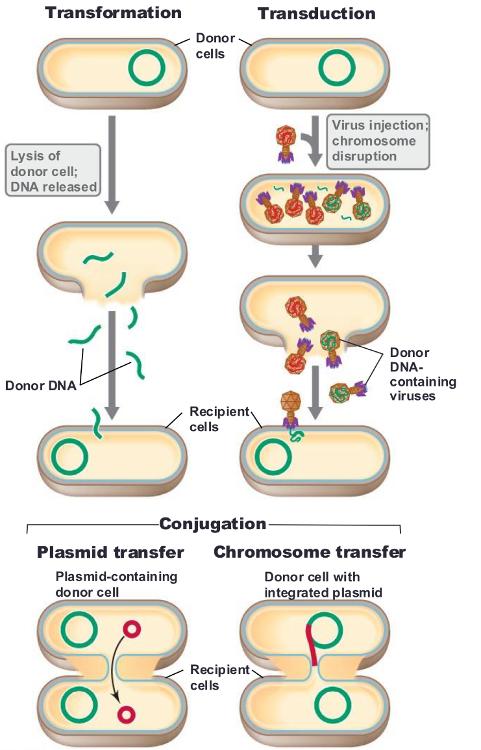 transformation transduction conjunction |
front 50 three possible fates of horizontal gene transfer | back 50 degradation replication recombination |
front 51 gene transfer in bacteria - genetic recombination recombination: | back 51 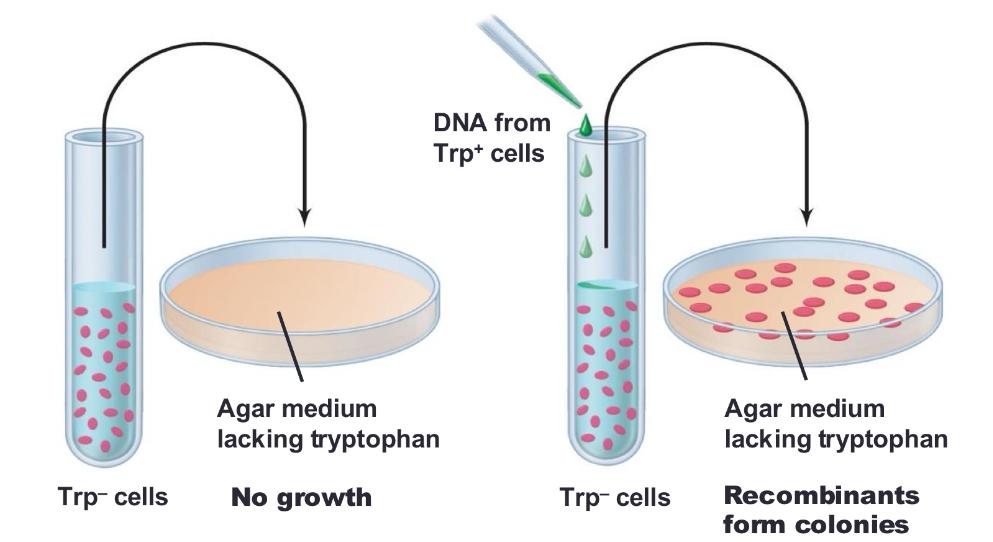 physical exchange of DNA between genetic elements |
front 52 homologous recombination: | back 52 process that results in genetic exchange between homologous DNA from two different sources. recA is essential. facilitating genetic variation and the repair of DNA damage |
front 53 selective medium can be used to ____ | back 53 detect rare genetic recombinants |
front 54 a simplified version of homologous recombination (video) | back 54 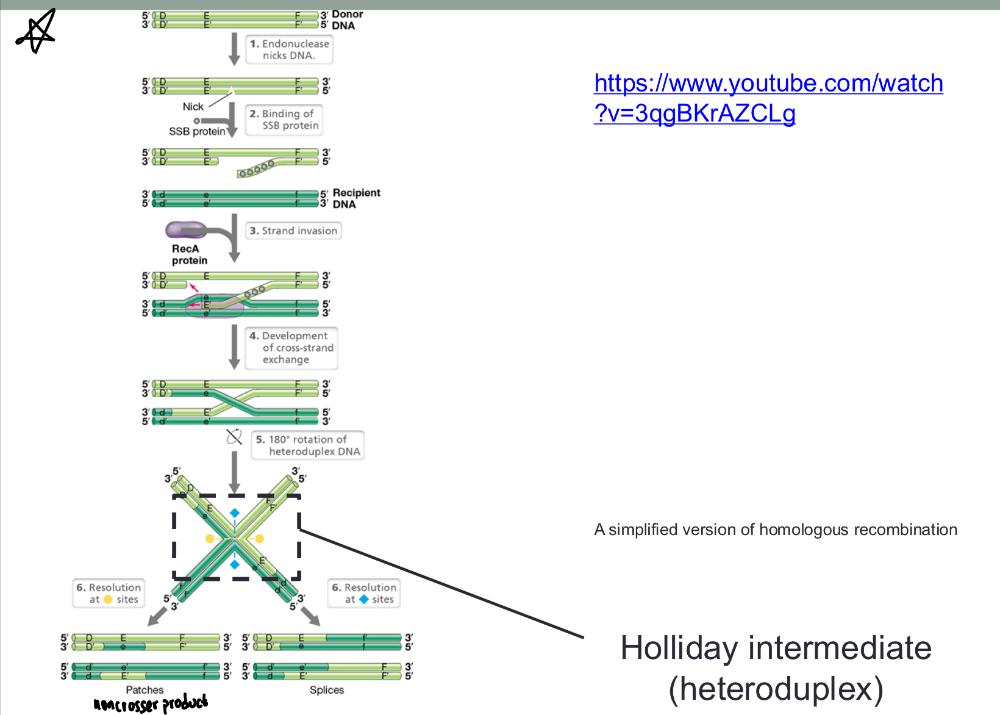 |
front 55 transformation | back 55 genetic transfer process by which DNA is incorporated into a recipient cell and brings about genetic change |
front 56 competent cells (recipient cell) | back 56 cells are capable of taking up DNA and being transformed
|
front 57 natural transformation of gram + by single-strand DNA (photo) | back 57 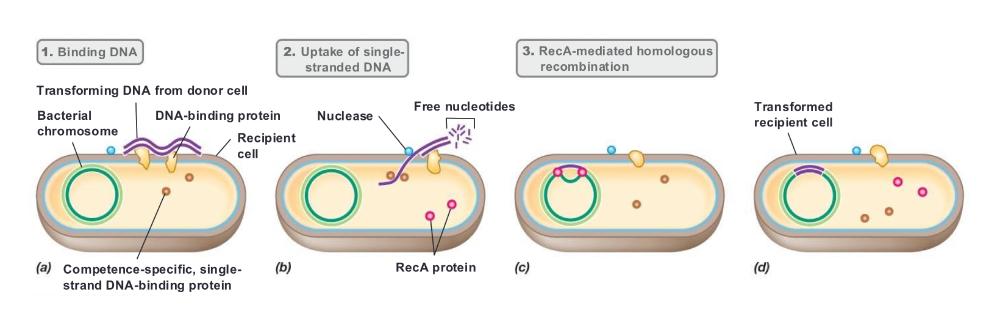 |
front 58 transduction | back 58 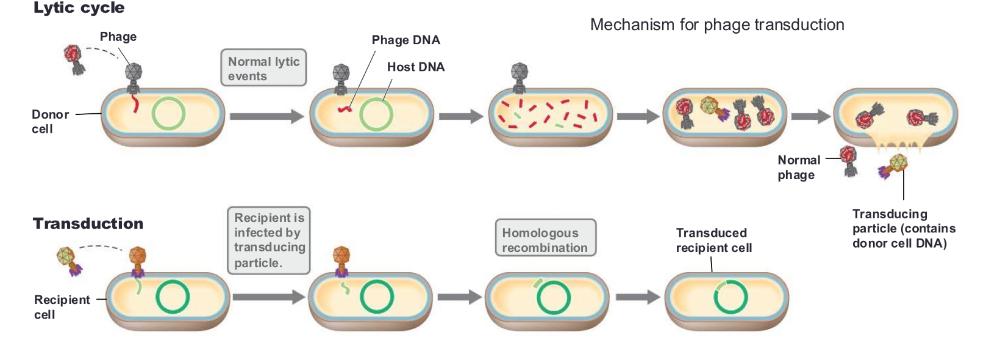 transfer of DNA from one cell to another by a bacteriophage two modes: generalized transduction and specialized transduction |
front 59 generalized transduction | back 59 DNA derived from virtually any portion of the host genome is packaged inside the mature virion
|
front 60 specialized transduction | back 60 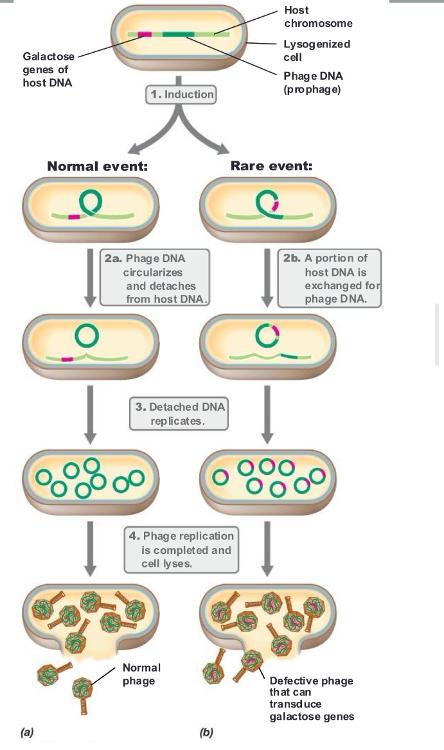 DNA from a specific region of the host chromosome is integrated directly in the virus genome
|
front 61 phage conversion | back 61 alteration of the phenotype of a host cell by a lysogenization (phage DNA incorporates into bacterial chromosome and becomes dormant)
|
front 62 bacterial conjugation (mating) | back 62 mechanism of genetic transfer that involces cell-to-cell contact
|
front 63 F(fertility) plasmid | back 63
|
front 64 DNA synthesis is necessary for ___ | back 64 DNA transfer by conjugation -DNA synthesized by rolling circle replication |
front 65 images of F-pilus | back 65 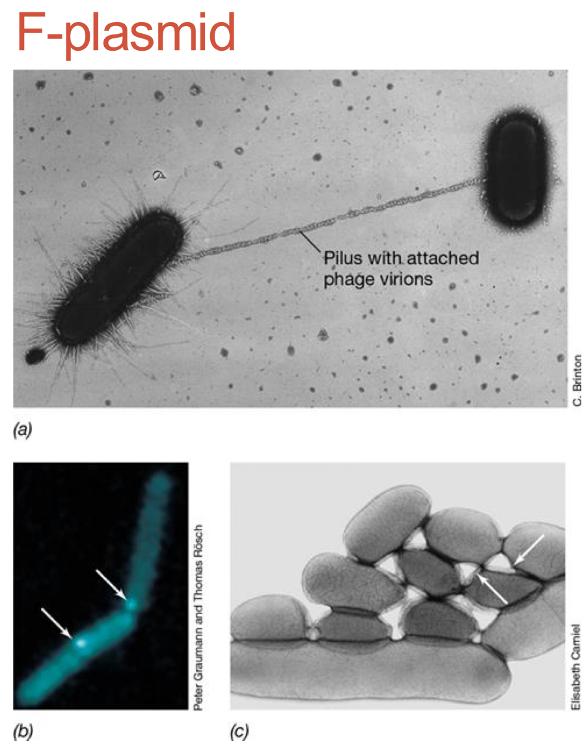 |
front 66 transfer of plasmid DNA by conjugation (photo) | back 66 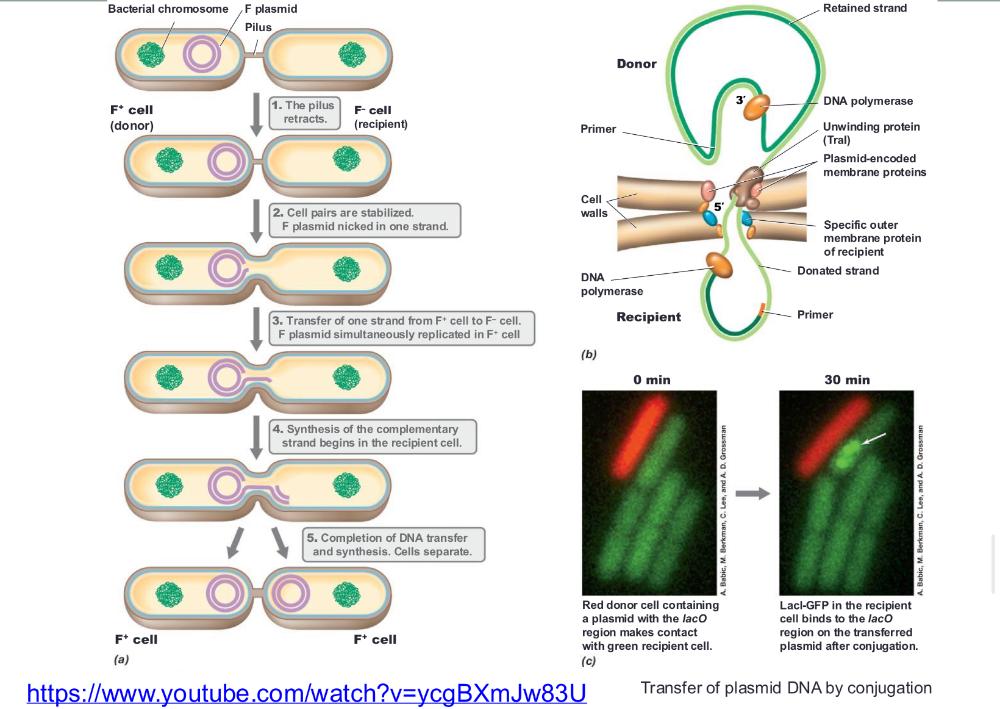 |
front 67 F plamids can integrate into ___ | back 67 host chromosome |
front 68 cells possesing a nonintegrated F plasmid are called ___ | back 68 F+ |
front 69 cells possessing an integrated F plasmid are called ___ | back 69 Hfr (high frequency of recombination)
|
front 70 presence of the F plasmid results in ____ | back 70 alterations in cell properties
|
front 71 insertion sequences (mobile elements) are ___ | back 71 present in both F plasmid and E coli chromosome, which facilitate homologous recombination plasmid is now part of chromosome. chromosomal genes transferred with plasmid |
front 72 the formation of an Hfr strain (photo) | back 72 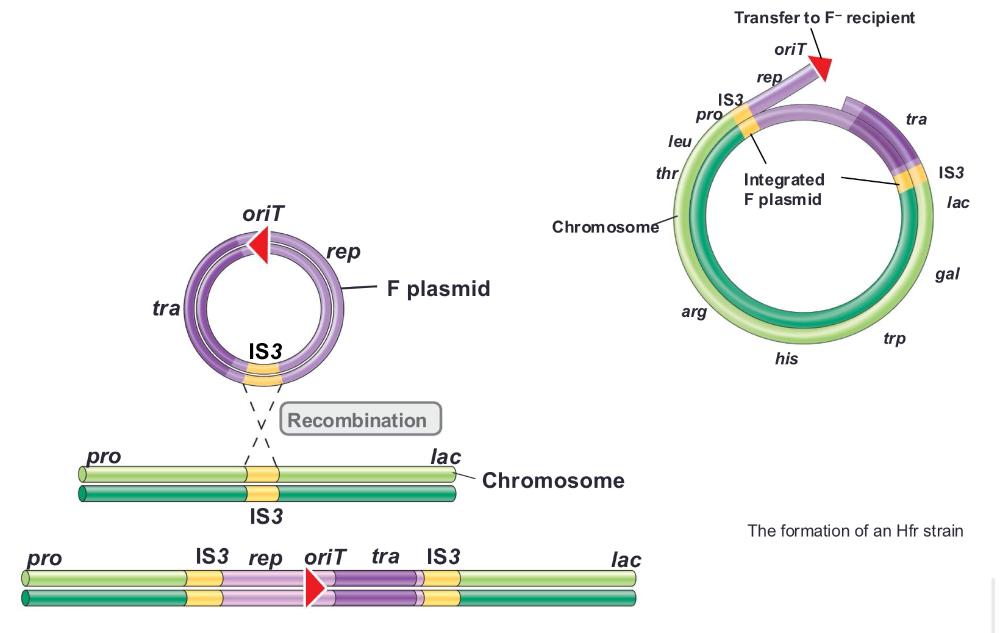 |
front 73 recipient cell remains as F- and does NOT become Hfr because ____ | back 73 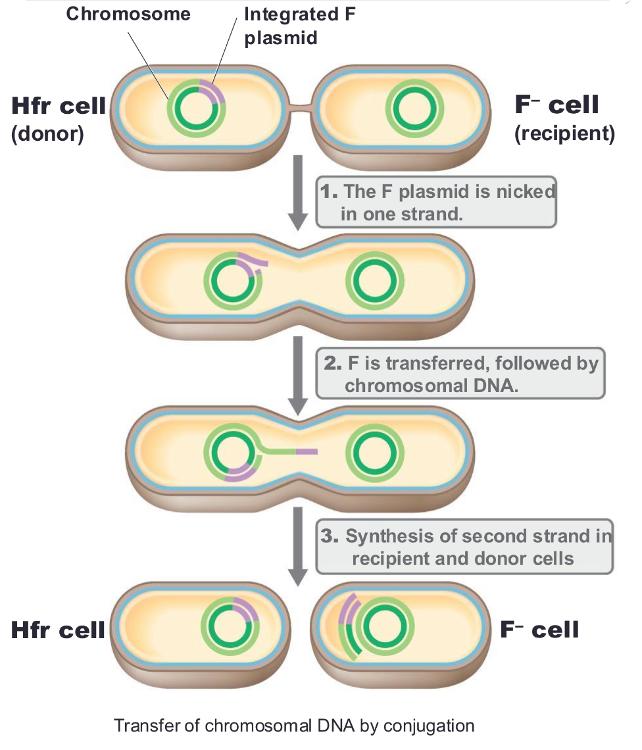 only a portion of the integrated F plasmid is transferred by the donor |
front 74 discrete segemnts of DNA that move as a unit from one location to another within other DNA molecules are _____ | back 74 transposable elements |
front 75 transposable elements can be found in all ___ | back 75 three domains of life move by a process called transposition
|
front 76 transposable elements: Two main types of transposable elements in Bacteria are ____ and ____. | back 76 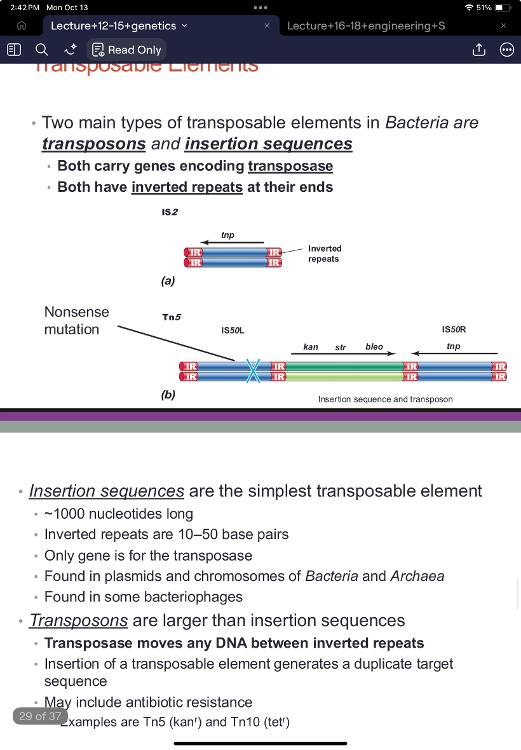 transposons and insertions sequences
|
front 77 insertion sequences | back 77 are the simplest transposable element
only encode one protein |
front 78 transposons | back 78 are larger than insertion sequences
- ex. Tn5 (kanr) and Tn10 (tetr) often times carry useful genes major player in the spread of antibiotic resistancy |
front 79 transposition replicates target sequence (photo) | back 79 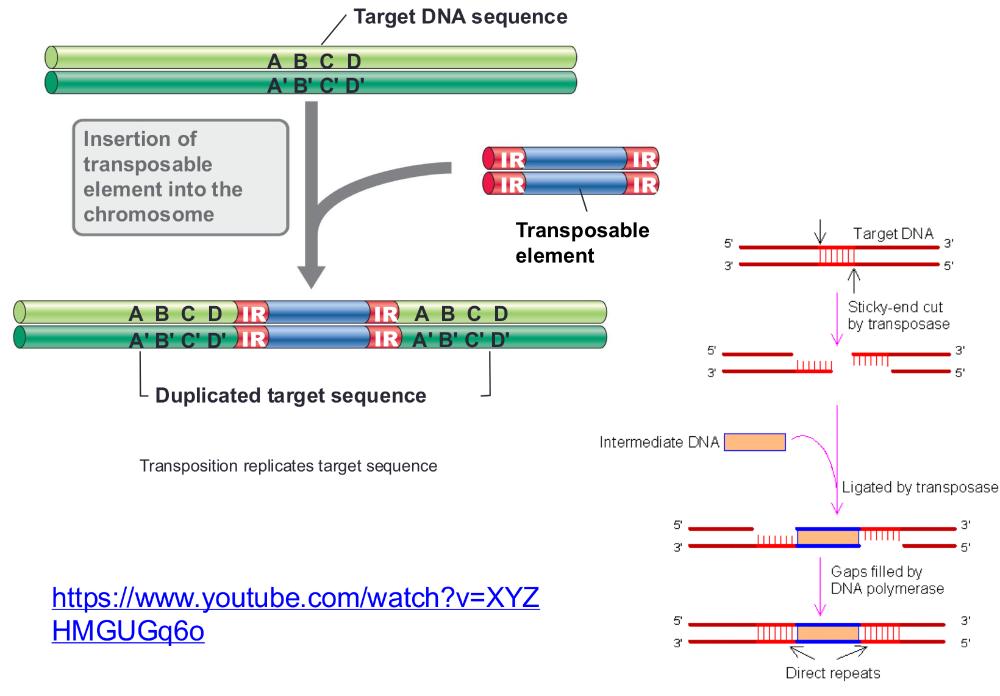 |
front 80 using transposons to make mutants: | back 80 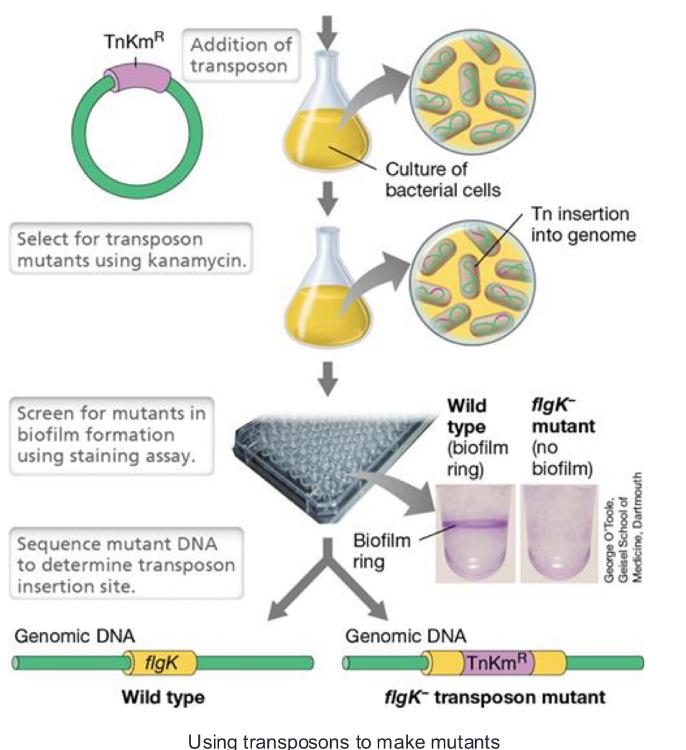
|
front 81 Preserving Genome Integrity: CRISPR Interference CRISPR: ____ | back 81 Clustered Regulatory Interspaced Short Palindromic Repeats Type of prokaryotic "immune system"
• CRISPR-associated proteins (Cas proteins)
|
front 82 Genome Editing and CRISPRs • Sequence targeting by the Cas9 protein | back 82
|
front 83 Operation of the CRISPR system (photo) | back 83 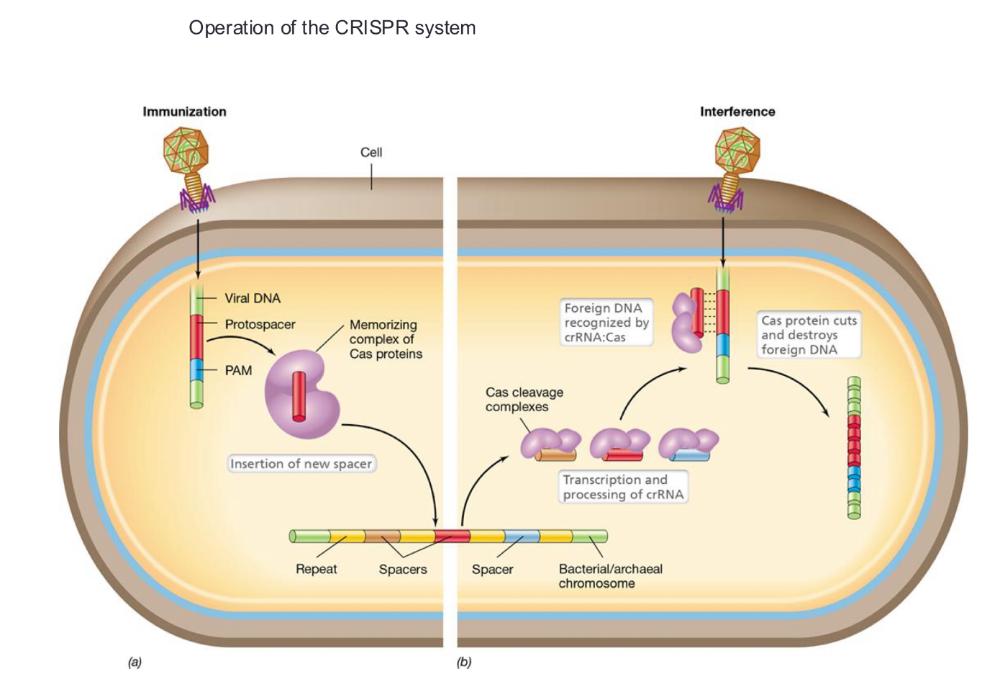 |
front 84 CRISPRs and genome editing (photo) | back 84 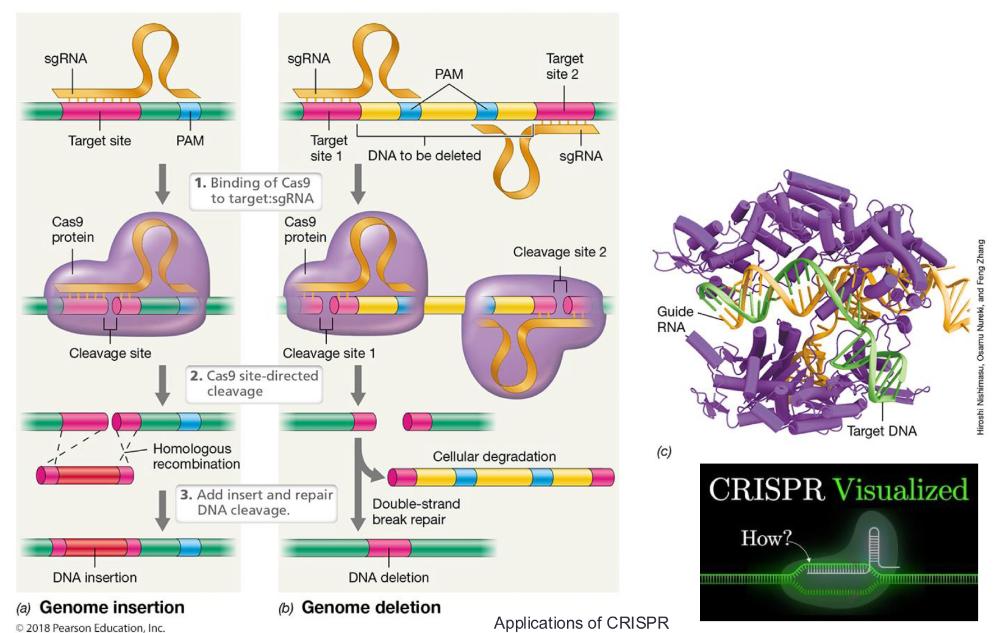 |
front 85 The applications of CRISPR-Cas9 (photo) | back 85 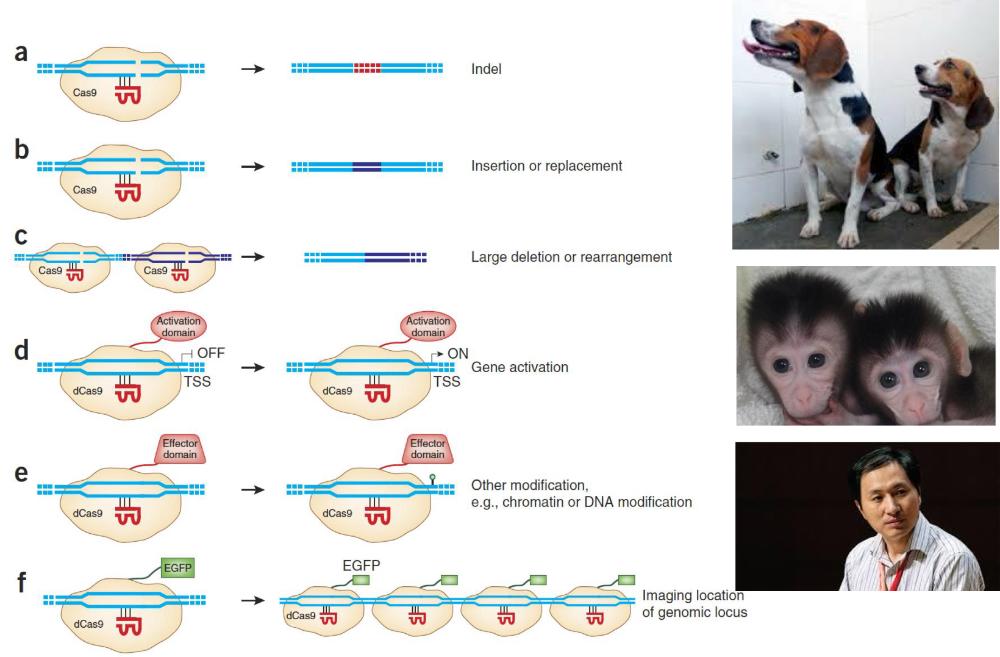 |