Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Biology IGCSE part 1
front 1 Describe the characteristics of living organisms | back 1 (a) movement as an action by an organism or part of an organism causing a change of position or place (b) respiration as the chemical reactions in cells that break down nutrient molecules and release energy for metabolism (c) sensitivity as the ability to detect and respond to changes in the internal or external environment (d) growth as a permanent increase in size and dry mass (e) reproduction as the processes that make more of the same kind of organism (f) excretion as the removal of the waste products of metabolism and substances in excess of requirements (g) nutrition as the taking in of materials for energy, growth and development |
front 2 species definition | back 2 A group of organisms that can reproduce to produce fertile offspring. |
front 3 binomial system definition | back 3 An internationally agreed system in which the scientific name of an organism is made up of two parts showing the genus and species. |
front 4 binomial system order and components | back 4
|
front 5 Explain classification systems | back 5 Explain that classification systems aim to reflect evolutionary relationships. Explain that the sequences of bases in DNA are used as a means of classification. Groups of organisms which share a more recent ancestor (are more closely related) have base sequences in DNA that are more similar than those that share only a distant ancestor. |
front 6 Classes of vertebrates and definition | back 6  Animals that have a vertebral column (spine). |
front 7 Features of mammals. | back 7  |
front 8 Features of fish | back 8  |
front 9 Features of reptiles | back 9  |
front 10 Features of birds | back 10  |
front 11 Features of amphibians | back 11  |
front 12 Arthropods classes and definition. | back 12  Animals that don't have a vertebral column (spine). |
front 13 Parts of arthropods body (exceptions) | back 13 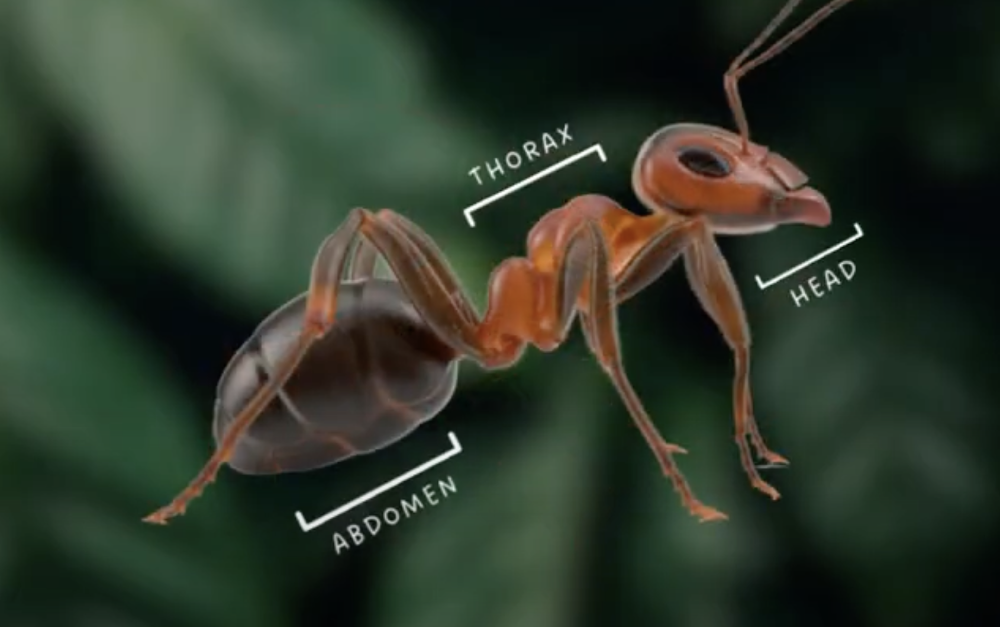 Head, thorax and abdomen |
front 14 Features of insects | back 14  |
front 15 Features of arachnids | back 15  |
front 16 Features of crustaceans | back 16  |
front 17 Features of myriapods | back 17  |
front 18 Five kingdoms | back 18 Animal, plants, fungus, prokaryote, protoctist |
front 19 Features of animal kingdom | back 19  |
front 20 Features of the plant kingdom | back 20  |
front 21 Features of fungi kingdom | back 21  |
front 22 Features of prokaryote kingdom | back 22  |
front 23 Features of protoctist kingdom | back 23  |
front 24 Features of viruses | back 24  |
front 25 Features of ferns | back 25  |
front 26 Features of monocotyledons | back 26  |
front 27 Features of dicotyledons | back 27  |
front 28 Describe and compare the structure of a plant cell with an animal cell | back 28 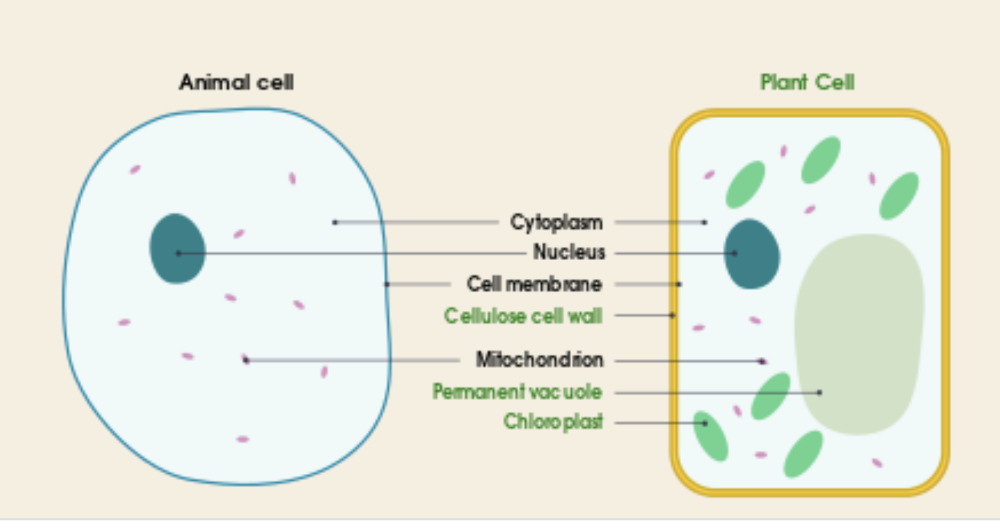 Similarities:
Differences:
|
front 29 Bacterial cell describe parts | back 29 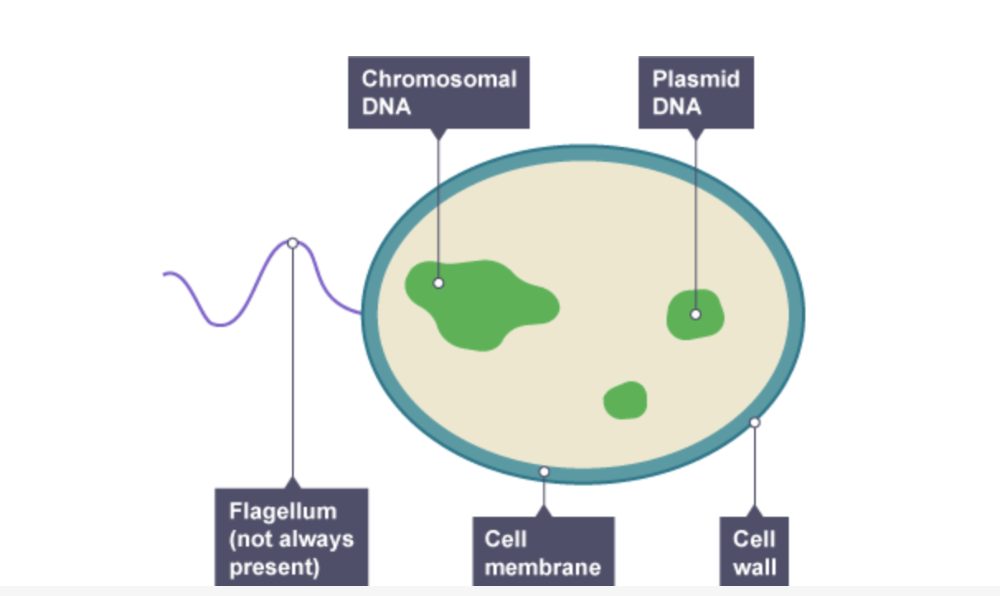
|
front 30 Cell Wall functions in plants and bacteria separate | back 30 Plant
Bacteria
|
front 31 Cell membrane functions all cells | back 31
|
front 32 Nucleus functions plants and animals | back 32
|
front 33 Cytoplasm functions all Cells | back 33
|
front 34 Chloroplasts functions | back 34
|
front 35 Ribosomes functions all cells | back 35
|
front 36 Mitochondria functions plant and animal | back 36 Produce the energy-carrying molecule ATP (adenosine triphosphate). This process releases energy from glucose, fueling various cellular activities. |
front 37 vacuole cell functions, animal and plant | back 37
|
front 38 Circular DNA and Plasmids in bacteria functions | back 38
|
front 39 How are new cells produced? | back 39 New cells are produced by division of existing cells |
front 40 State that specialised cells have specific functions, limited to: | back 40 (a) ciliated cells – movement of mucus in the trachea and bronchi (b) root hair cells – absorption (c) palisade mesophyll cells – photosynthesis (d) neurones – conduction of electrical impulses (e) red blood cells – transport of oxygen (f) sperm and egg cells (gametes) – reproduction |
front 41 Cell definition | back 41 Basic building block of all living organisms. |
front 42 Tissue | back 42 A group of specialised cells working together to carry out a specific function. |
front 43 Organ | back 43 A group of specialised tissues working together to carry out a specific function. |
front 44 Organ system | back 44 A group of specialised organs working together to carry out a specific function. |
front 45 Organsim | back 45 A living thing. |
front 46 magnification formula | back 46 magnification = image size ÷ actual size |
front 47 Convert between millimetres (mm) and micrometres (μm) | back 47 1 millimetre (mm) = 1,000 micrometres (μm) |
front 48 Diffusion definition | back 48 The net movement of particles from a region of their higher concentration to a region of their lower concentration (i.e. down a concentration gradient), as a result of their random movement. Happens due to kinetic energy of particles causing random movement. |
front 49 Investigate the factors that influence diffusion | back 49 Surface area: the larger the surface area, the more space there is for particles to diffuse across, increasing the rate of diffusion. Temperature: higher temperatures give particles more energy, allowing them to move faster. This increases the rate of diffusion. Concentration gradients: The steeper the concentration gradient, the greater the difference in concentrations. This means, more particles will move from their region of higher concentration to their region of lower concentration to achieve equilibrium, thereby increasing the rate of diffusion. Diffusion distance: this is the distance that particles have to travel to achieve equilibrium. The smaller the diffusion distance, the less time it takes to achieve equilibrium, so higher the rate of diffusion. |
front 50 Osmosis definition | back 50 Describe osmosis as the net movement of water molecules from a region of higher water potential (dilute solution) to a region of lower water potential (concentrated solution), through a partially permeable membrane. |
front 51 Describe the role of water as a solvent in organisms | back 51 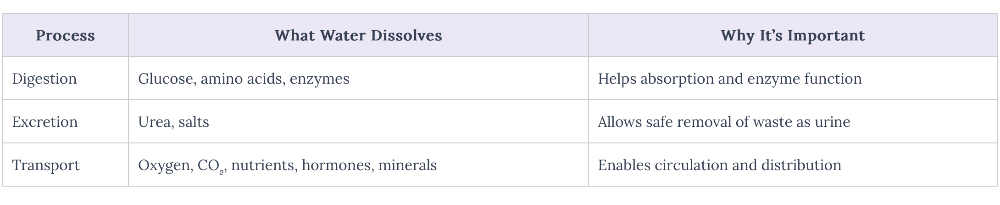 |
front 52 Investigate osmosis using materials such as... | back 52 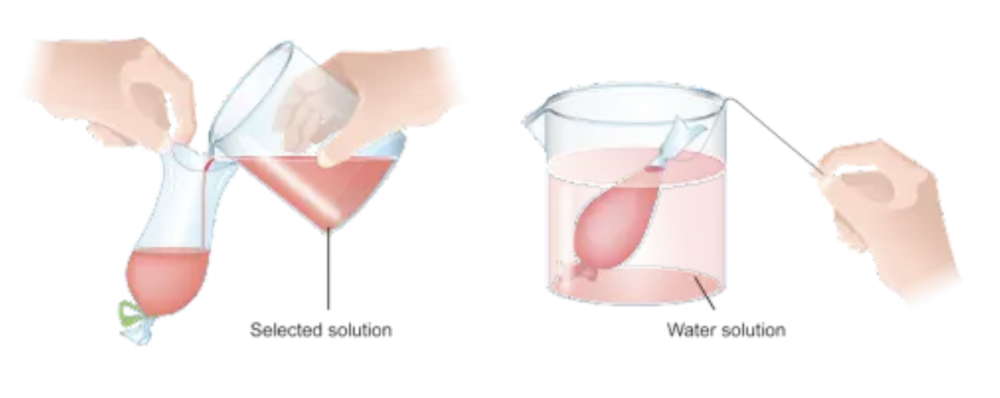
Change in volumes or mass shows what way osmosis went. |
front 53 Explain the effects on plant cells of immersing them in solutions of different concentrations | back 53 The pressure water applies in plants is turgor pressure and turgidity is the state of being ‘turgid'. Plants need turgid cells to help them maintain their shape and in turn, help the plant stay upright. Water is mainly stored in the vacuole in the cytoplasm, and it is mainly this vacuole that regulates the turgidity of a plant cell.
Water diffuses out of the cell by osmosis. This causes the cytoplasm to shrink, and thus the cell membrane gets ripped away from the cell wall. This process is called plasmolysis. Cells become weak and flaccid, as there isn’t enough cytoplasm to support the cell and help it maintain its shape.
Since the concentration of the solution is equal inside and outside of the plant cells, there is no net movement of water. This means the volume or shape of the plant cell is unlikely to change.
Water diffuses down its concentration gradient into the cell, by osmosis. This causes the amount of cell matter inside the cell to increase. As the cytoplasm enlarges, it pushes outwards on the cell surface membrane more and more. Normally, this would usually cause the cell surface membrane to eventually burst (once the turgor pressure grows too large). However, plant cells have very strong cell walls. This holds the plant cell intact, and as the cytoplasm pushes outside, the cell simply swells to its full size and becomes rigid. This cell is turgid. |
front 54 Describe active transport | back 54 The movement of particles through a cell membrane from a region of lower concentration to a region of higher concentration (i.e. against a concentration gradient), using energy from respiration. |
front 55 What move molecules or ions across a membrane during active transport | back 55 protein carriers |
front 56 examples of active transport | back 56
|
front 57 List the chemical elements that make up carbohydrates | back 57 Carbon, Hydrogen and Oxygen. |
front 58 List the chemical elements that make up fats | back 58 Carbon, Hydrogen and Oxygen. |
front 59 List the chemical elements that make up proteins | back 59 Carbon, Hydrogen and Oxygen, and Nitrogen. sometimes sulfur and phosphorus too |
front 60 What are starch, glycogen and cellulose made from | back 60 glucose |
front 61 What are proteins made from | back 61 amino acids |
front 62 What are fats and oils made from | back 62 fatty acids and glycerol |
front 63 Describe the use of: Iodine solution to test for starch | back 63 Add a few drops of iodine to the test solution/ test material. If it contains starch, the solution will turn blue-black, if not, it’ll remain an orangey/ brown colour (the colour of iodine). |
front 64 Describe the use of Benedict’s solution to test for reducing sugars | back 64 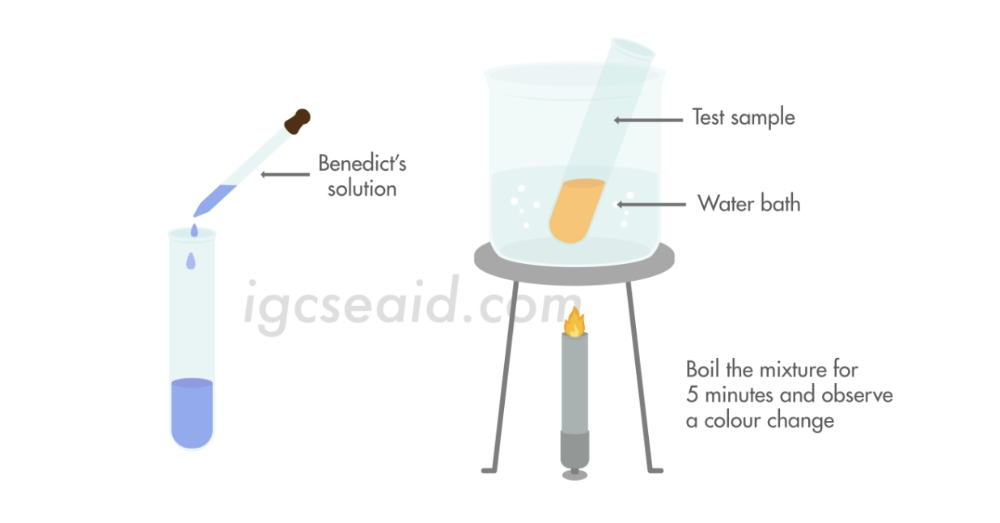 To a known volume of test solution, add the equal volume of Benedict’s reagent/ solution. Give it a stir and look for any colour changes. (If none, try heating it in a warm water bath (about 80oC), and look for any colour changes.) If stays blue, there are no reducing sugars present . If there are any sugars present, it’ll change from blue to green, to yellow, to orange, to red. Green means that there are very low reducing sugars and red the most. Note: sucrose is not a reducing sugar. |
front 65 Describe the use of Biuret test for proteins | back 65 Biuret reagent is a mix of two chemicals – copper sulfate (CuSO4) mixed with either sodium hydroxide (NaOH) or potassium hydroxide (KOH). To perform the test, simply add the biuret reagent to the test solution. (Note, if the test material is solid and not liquid, crush it and mix it with distilled water, to form a solution). 1:1 ratio of solution and reagent. If peptide bonds are present, the blue reagent will turn mauve or purple. Measure out a known volume of test solution into a test tube. About 1cm3 should be enough. Add the same volume of NaOH (or KOH) to the test tube and stir. Add a few drops of CuSO4 solution, shaking after each drop. If buret reagent not already pre made |
front 66 Describe the use of Ethanol emulsion test for fats and oils. | back 66 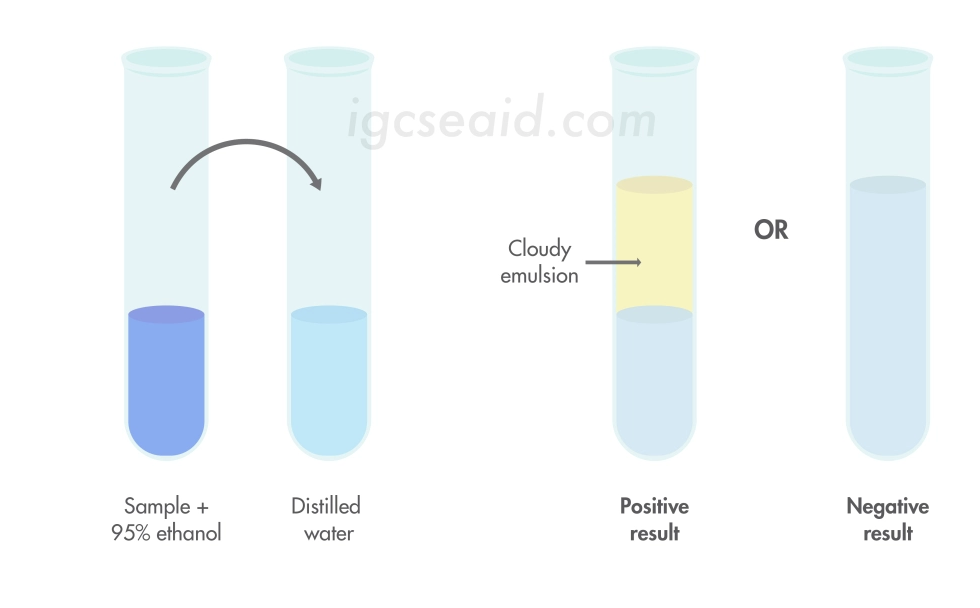 Add the test sample to a concentrated ethanol solution. You put the mixture into a test tube of distilled water, close it, and shake it around. If a cloudy emulsion forms, fats are present; if not, there are no fats. |
front 67 DCPIP test for vitamin C | back 67 1. Prepare the DCPIP solution: Dissolve DCPIP powder in distilled water to create a blue solution. 2. Prepare the sample:If the sample is a solid it needs to be ground or crushed and mixed with distilled water to form a solution. 3. Titration:Add the sample solution dropwise to a fixed volume of the blue DCPIP solution in a test tube. 4. Observe the color change: Swirl the test tube gently after each drop. The blue color of the DCPIP will fade and eventually disappear (become colorless) when enough vitamin C has been added to reduce all the DCPIP. 5. Determine the concentration: The lower the volume of sample needed, the higher the vitamin C concentration in that sample. |
front 68 pH and the use of hydrogencarbonate indicator, litmus and universal indicator | back 68 Litmus - Red in acid, blue in alkaline.
|
front 69 methylene blue dye | back 69 In IGCSE Biology, methylene blue dye is used as an indicator to measure the rate of aerobic respiration in living cells like yeast. As the yeast respire, they release hydrogen ions that reduce the dye, causing it to change from blue to colorless. The time it takes for this color change to occur is a measure of the respiration rate; a shorter time indicates a faster rate. |
front 70 sodium hydrogencarbonate (sodium bicarbonate) | back 70  |