Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Acids and Bases 2
front 1 HCl | back 1  Strong Molecular Acid Fully Dissociates |
front 2 HBr | back 2 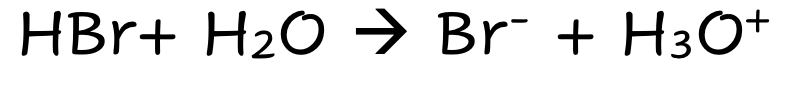 Strong Molecular Acid Fully Dissociates |
front 3 HNO3 | back 3 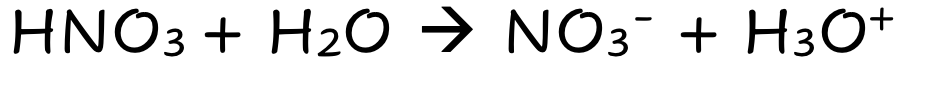 Strong Molecular Acid Fully Dissociates |
front 4 CH3COOH | back 4 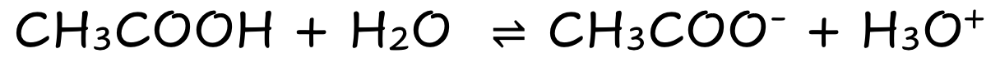 Weak Molecular Acid Partially Dissociates |
front 5 HF | back 5 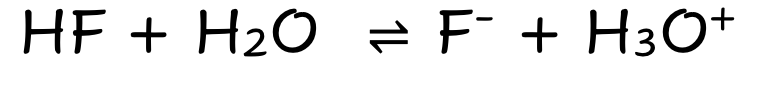 Weak Molecular Acid Partially Dissociates |
front 6 NH3 | back 6 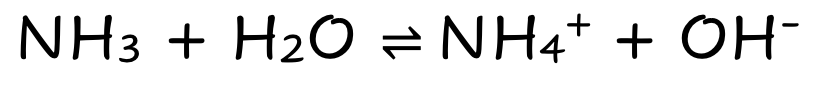 Weak Molecular Base Partially Dissociates |
front 7 KOH | back 7 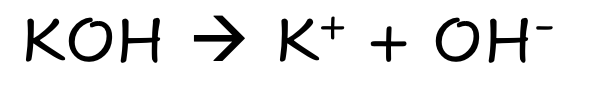 Strong Ionic Base Fully Dissolves |
front 8 NaOH | back 8  Strong Ionic Base Fully Dissolves |
front 9 NaCl | back 9  Neutral Ionic Salt Fully Dissolves |
front 10 NH4Cl | back 10 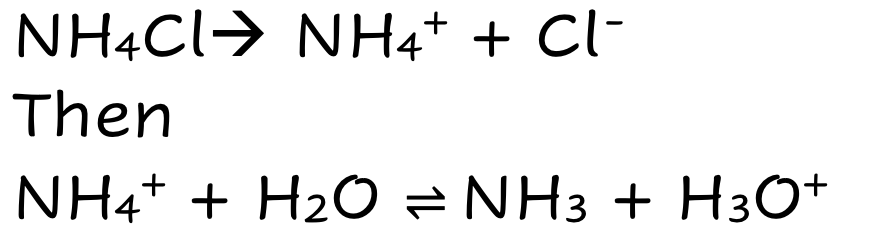 Weak Ionic Acid Fully Dissolves THEN Partially Dissociates |
front 11 CH3COONa | back 11 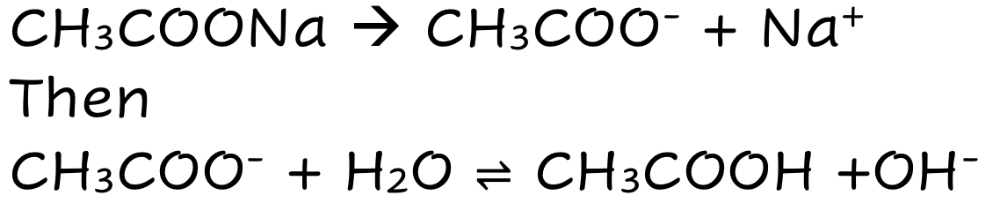 Weak Ionic Base Fully Dissolves THEN Partially Dissociates |
front 12 CH3CH2OH | back 12 Molecular and Neutral Disolves in water. |