Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
BMD 320 Exam 2 Study Guide Questions
front 1 What are the functions of the nucleus and nuclear envelope? | back 1 The nucleus stores genetic material and controls gene expression. The nuclear envelope regulates molecular traffic between nucleus and cytoplasm. |
front 2 What is the nucleolus and its three regions? | back 2 The nucleolus is the site of ribosome biogenesis. It has three regions: Fibrillar Centers (rRNA genes), Dense Fibrillar Component (active transcription), and Granular Component (ribosome assembly). |
front 3 What is ribosome biogenesis? | back 3 making protein factories inside the cell so it can make proteins |
front 4 Describe the fibrillar centers | back 4 ribosomal RNA gene zones inside the nucleolus where rRNA is first made |
front 5 Describe Dense Fibrillar Component | back 5 processing factory in the nucleolus where rRNA is cleaned up and prepared |
front 6 Describe Granular Component | back 6 ribosome assembly area where ribosomal parts come together before leaving the nucleolus |
front 7 Name three nuclear substructures and their functions | back 7 PML bodies (tumor suppression), Cajal bodies (snRNP maturation), Speckles (mRNA splicing). |
front 8 What is the nuclear lamina? | back 8 A fibrous layer providing structural support; composed of lamin proteins. |
front 9 What are consequences of lamin A mutations? | back 9 They cause nuclear fragility, blebbing, altered gene expression, diseases like Hutchinson-Gilford Progeria. "Blebbing" refers to the formation of bubble-like protrusions (called blebs) on the surface of the cell, typically involving the plasma membrane or nuclear envelope. It’s a physical sign that something is going wrong with cell structure or function. Hutchinson-Gilford Progeria Syndrome (HGPS) is a rare genetic disorder characterized by dramatically accelerated aging in children. It’s one of the most well-studied examples of how defects in nuclear structure can lead to disease. |
front 10 What is the function of nuclear pores? | back 10 They regulate import and export of molecules between nucleus and cytoplasm. |
front 11 Describe the structure of the nuclear pore complex. | back 11 It includes FG-repeat proteins that form a selective barrier. FG-repeat proteins are key components of the nuclear pore complex (NPC) that regulate what enters and exits the nucleus. |
front 12 How does nuclear import/export occur? | back 12 It involves Ran-GTP/Ran-GDP, importins, exportins, and NLS signals. Getting Into the Nucleus (Import)
Getting Out of the Nucleus (Export)
|
front 13 List the levels of DNA packaging. | back 13 DNA → nucleosomes → beads on a string → 30-nm fiber → solenoid → metaphase chromosome. Steps of DNA packing
|
front 14 What proteins form the nucleosome? | back 14 Histones: H2A, H2B, H3, and H4 (two of each). The nucleosome is the basic unit of DNA packaging in eukaryotic cells. It looks like "beads on a string" under a microscope. Each nucleosome core is made up of 8 histone proteins:
Together, these form a histone octamer |
front 15 Name parts of a mitotic chromosome. | back 15
|
front 16 What is junk DNA? | back 16 Non-coding DNA including introns, transposons, and repetitive elements.
|
front 17 What do cohesins and condensins do? | back 17 Cohesins hold sister chromatids together; condensins compact DNA. |
front 18 What triggers nuclear envelope breakdown in mitosis? | back 18 Phosphorylation of lamins. |
front 19 Define euchromatin and heterochromatin. | back 19 Euchromatin is loosely packed and active; heterochromatin is dense and silent. |
front 20 What is epigenetics? Give an example. | back 20 Heritable changes in gene expression without DNA sequence change. Example: X-inactivation (Barr body). |
front 21 How do histone modifications regulate genes? | back 21 They affect chromatin structure and transcription factor access. |
front 22 List the phases of the cell cycle. | back 22
|
front 23 What are the cell cycle checkpoints? | back 23 G1 (DNA damage), S (replication), G2 (DNA replication), M (spindle checkpoint). |
front 24 How do cyclins and Cdks regulate the cell cycle? | back 24 By phosphorylation, synthesis, and degradation of target proteins. |
front 25 What is the function of the APC/C complex? | back 25 It targets proteins like cyclin B for degradation via ubiquitination. Ubiquitination: A process where a small protein called ubiquitin is attached to a target protein, marking it for degradation by the proteasome |
front 26 Name three CKIs and their roles. | back 26 p21, p27, p16: inhibit cyclin-dependent kinases (Cdks) and help control cell cycle progression by slowing or stopping the cycle when needed |
front 27 What is the G0 phase? | back 27 A resting state where cells are not dividing. |
front 28 What is the G1 restriction point? | back 28 A checkpoint deciding cell fate based on nutrients, growth factors, and DNA integrity. |
front 29 What role does p53 play in the cell cycle? | back 29 It halts the cycle in response to DNA damage. |
front 30 What is the key event in S phase? | back 30 DNA replication. |
front 31 Describe DNA replication enzymes. | back 31 Helicase: Unzips the DNA double helix by breaking hydrogen bonds between base pairs, creating the replication fork. Primase: Synthesizes short RNA primers that provide a starting point for DNA polymerase. DNA Polymerase: Adds new nucleotides to the 3’ end of the primer to synthesize the new DNA strand. Ligase: Joins Okazaki fragments on the lagging strand by sealing nicks in the sugar-phosphate backbone. Topoisomerase: Relieves tension and prevents supercoiling ahead of the replication fork by making temporary cuts in the DNA. SSBPs (Single-Strand Binding Proteins): Bind to and stabilize single-stranded DNA after helicase unwinds it, preventing the strands from re-annealing. |
front 32 What are telomeres and telomerase? | back 32 Telomeres protect chromosome ends; telomerase extends them |
front 33 How is DNA damage repaired? | back 33 Through proofreading, base/nucleotide excision, and mismatch repair |
front 34 What regulates the G2/M transition? | back 34 Cdc25C activates Cdk1-cyclin B by removing inhibitory phosphates; Wee1 inhibits it. |
front 35 How does Cdk1 activation trigger mitosis? | back 35 It initiates positive feedback loops and phosphorylates proteins needed for mitosis |
front 36 What are the stages of mitosis? | back 36 Prophase, Prometaphase, Metaphase, Anaphase, Telophase, Cytokinesis |
front 37 What happens in Prophase? | back 37 Chromosomes condense, centrosomes move to poles, spindle forms. |
front 38 What occurs in Prometaphase? | back 38 Nuclear envelope breaks down, microtubules attach to kinetochores |
front 39 What is the key event of Metaphase? | back 39 Chromosomes align at the metaphase plate or in the middle; Cyclin B and securin are degraded |
front 40 What happens during Anaphase? | back 40 Cohesin is cleaved, chromatids separate via dynein and microtubules |
front 41 What happens in Telophase? | back 41 Nuclear envelope reforms, chromosomes decondense. |
front 42 What occurs during Cytokinesis? | back 42 Actin and myosin filaments form a contractile ring to split the cell |
front 43 What happens to the Golgi during mitosis? | back 43 It fragments and reassembles in daughter cells. |
front 44 What is the structure of the mitochondrion? | back 44 Outer Membrane
Intermembrane Space
Inner Membrane
Cristae
Matrix
|
front 45 How is mitochondrial DNA inherited? | back 45 Maternally, through the egg cytoplasm |
front 46 What is heteroplasmy? | back 46 Presence of more than one type of mitochondrial DNA in a cell |
front 47 What macronutrients are metabolized for energy? | back 47 Glucose, fatty acids, amino acids |
front 48 What are key steps in glucose metabolism? | back 48 1. Glycolysis
2. Pyruvate → Acetyl-CoA
3. The Citric Acid Cycle (Krebs Cycle)
4. Electron Transport Chain (ETC)
Total ATP (per glucose): About 32 ATP |
front 49 What are the key steps in amino acid and lipid metabolism? | back 49 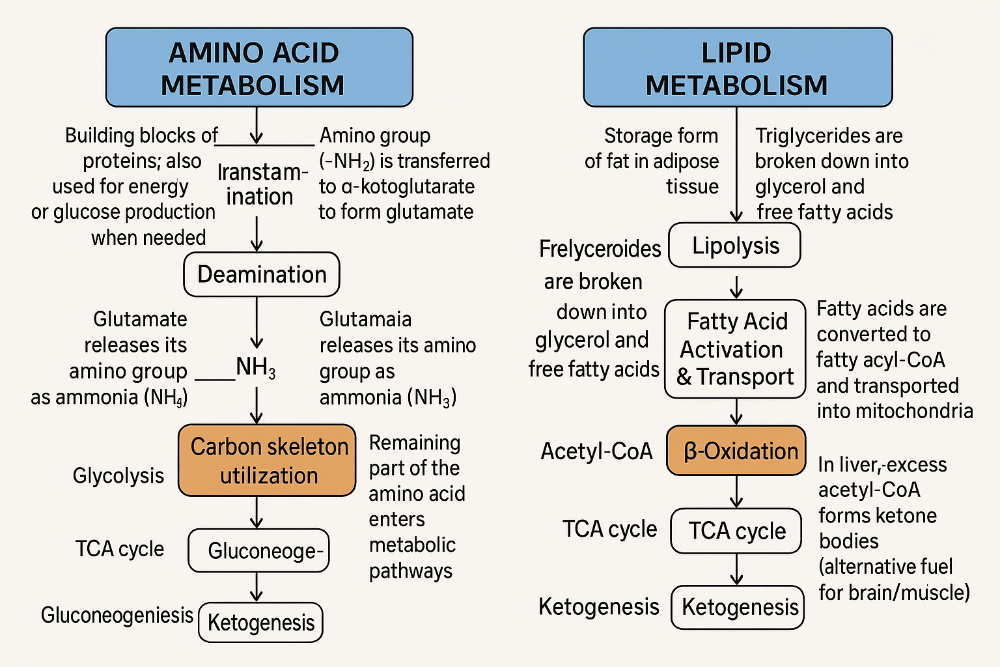 |
front 50 What is the proton motive force? | back 50 Electrochemical gradient that drives ATP synthesis. |
front 51 What is the role of oxygen in respiration? | back 51 Final electron acceptor in the electron transport chain. |
front 52 How is ATP synthesized in mitochondria? | back 52 H+ gradient drives ATP synthase in oxidative phosphorylation. |
front 53 How do mitochondria balance ATP, heat, and ROS ( Reactive Oxygen Species? | back 53 Uncoupling proteins shift energy from ATP to heat, lowering ROS |
front 54 What are the three main cytoskeletal proteins? | back 54 Intermediate Filaments Job: Give the cell strength and hold things in place Strong and rope-like Found in: Skin, hair, and around the nucleus Microtubules Job: Act like train tracks for moving things around the cell Hollow tubes Help with: Cell division, cilia/flagella movement Built from: Tubulin Grow from the centrosome Actin Filaments (Microfilaments) Job: Help the cell move, change shape, and divide Thin and flexible Built from: Actin |
front 55 Describe intermediate filaments. | back 55 Shape: Rope-like •Function: Give the cell tensile strength (helps resist stretching and pulling) Made of: Keratin (skin, hair, nails) Vimentin (connective tissue) Lamins (support the nuclear envelope) Key Point:They hold the cell together and keep the nucleus stable. |
front 56 Name diseases from intermediate filament mutations. | back 56 Epidermolysis Bullosa Simplex (EBS) skin disease where the skin is very fragile. Even a little rubbing or bump can cause blisters. It's caused by problem with certain skin proteins. Usually runs in families. No cure, but you can take care of the skin to avoid problems. Progeria: very rare disease where kids age much faster than normal. Caused by a mistake in a gene that keeps the cell's "control center" strong. Kids look old early, with thin skin, hair loss, and heart problems. Usually happens by chance, not inherited. No cure, doctors help manage symptoms. |
front 57 Describe microtubule structure. | back 57 Tiny hollow tubes inside cells made of two proteins called alpha and beta tubulin. They have a "fast" end (plus) that grows or shrinks quickly. They have a "slow" end (minus) that changes slowly. They help the cell keep its shape and move things inside. |
front 58 What is dynamic instability? | back 58 Rapid switching between growth and shrinkage of microtubules. |
front 59 What are microtubules used for? | back 59 Spindle formation, transport, cilia and flagella movement. |
front 60 What do kinesin and dynein do? | back 60 They are like tiny trucks inside cells that carry stuff (cargo). Kinesin moves cargo toward the plus (+) end of microtubules (usually outward from the center). Dynein moves cargo toward the minus (-) end (usually inward toward the cell center). These proteins "walk" along microtubules to deliver materials where needed. |
front 61 Describe the ciliary axoneme structure. | back 61 The inside part of tiny cell hairs called cilia and flagella that helps them move. Made of 9 pairs of tubes around 2 single tubes in the middle (called the "9+2" pattern). Has motor parts called dynein arms that pulI on the tubes to make them bend. Special links hold the tubes together so they don't slide too far. Covered by the cell's outer membrane. |
front 62 What is Kartagener’s syndrome? | back 62 disease where tiny hairs in the body called cilia don't move right. This causes breathing problems and infections because mucus can't be cleared well. Some people's organs are flipped the other way (like the heart on the right side). It can also cause trouble having babies. Happens because the cilia's motors (dynein) don't work properly. |
front 63 Describe actin filaments. | back 63 The thinnest fibers inside cells. Made of a protein called actin that can join together (polymerize) when it has ATP attached. Very flexible and help cells change shape, move, and carry stuff inside. |
front 64 What is actin treadmilling? | back 64 Addition at plus end and removal at minus end |
front 65 What are steps of cell crawling? | back 65 Protrusion The cell sticks out its front (called lamellipodia) using actin. Adhesion The front of the cell grabs onto the surface. Traction The back of the cell pulls forward using actin and myosin (like tiny muscles) Actin pushes Cell grabs Myosin pulls. That's how the cell crawls forward! |
front 66 What regulates actin dynamics? | back 66 1. Rho proteins (Rho, Rac, Cdc42) tell the cell what actin shapes to make 2. Arp2/3 makes branched actin 3. Formin makes long, straight filaments 4. Cofilin cuts old actin to recycle it 5. Capping proteins stop actin from growing 6. Bundling/crosslinking proteins organize actin into structures |
front 67 How do actin and myosin interact in muscle? | back 67 Actin and myosin are proteins that slide past each other to make muscles contract. 1. Myosin grabs actin (using its "head" like a hook) 2. ATP gives energy Myosin uses ATP to move its head 3. Myosin pulls actin This shortens the muscle (contraction) 4. ATP binds again - Myosin lets go and resets for the next pull |
front 68 What is the ECM? | back 68 A network of proteins and polysaccharides providing structural and biochemical support. |
front 69 What cells contribute to the ECM? | back 69 Fibroblasts Make collagen and other fibers; help build and repair connective tissue Mesenchymal Stem Cells (MSCS) Can turn into bone, fat, muscle, or cartilage cells Immune Cells Defend the body (like crophages, T cells, B cells) Adipocytes Fat cells that store energy Osteoblasts Build bone by making the bone matrix |
front 70 What are major ECM components? | back 70 Collagen Strong fiber that gives tissue strength Elastin Stretchy fiber that lets tissue bounce back Proteoglycans Protein sugar combos that hold water and cushion tissues Hyaluronan (Hyaluronic acid) A big sugar molecule that makes tissues slippery and hydrated Adhesive glycoproteins Help cells stick to the ECM (e.g., fibronectin, laminin) |
front 71 Describe collagen structure | back 71 Triple helix Collagen is made of 3 chains twisted together Gly-X-Y The repeating building block (Glycine-any amino acid-usually Proline or Hydroxyproline) Needs vitamin C and oxygen For hydroxylation, a step needed to stabilize the helix |
front 72 How does collagen organization relate to tissue function? | back 72 Parallel fibers in tendons; mesh in skin for flexibility. |
front 73 What is elastin? | back 73 has hydrophobic regions (for recoil/ stretch) And hydrophilic regions (for stability and crosslinking) This pattern lets elastin stretch and snap back, like a rubber band |
front 74 Define proteoglycans and GAGs. | back 74 Proteoglycans proteins with long sugar chains called (glycosaminoglycan chains) GAGS attached GAGS are polysaccharides like heparan sulfate, chondroitin sulfate, and dermatan sulfate GAGS are negatively charged and hold lots of water, giving tissues cushioning and support |
front 75 What do adhesive glycoproteins do? | back 75 Connect ECM to cells and mediate signaling; e.g., fibronectin |
front 76 What is the basal lamina? | back 76 Thin ECM layer supporting epithelial cells, rich in laminin. |
front 77 What do MMPs do? | back 77 Degrade ECM during remodeling; regulated by TIMPs |
front 78 Name diseases linked to ECM defects | back 78 Osteogenesis Imperfecta bones break easily (bad collagen) Marfan Syndrome stretchy, loose tissues (bad elastin fibers) Ehlers-Danlos very stretchy skin and joints (collagen problem) Chondrodysplasia = bones and cartilage don't grow right (growth problem) |
front 79 Name the three types of cell junctions. | back 79 Occluding: seal Anchoring: hold Communicating: talk |
front 80 What are tight junctions? | back 80 Occluding Junction Function: Create a tight barrier between cells to stop leaks Made of: Claudins (main sealing proteins) proteins (connect claudins to the cell's cytoskeleton) |
front 81 What are adherens junctions? | back 81 Anchoring junction Use cadherins to stick cells together Cadherins connect to actin inside the cell Help cells hold on tight and keep shape |
front 82 What are desmosomes? | back 82 Desmosomes = strong spot welds They use cadherins to stick cells together and connect to intermediate filaments inside for strength |
front 83 What are focal adhesions? | back 83 Focal adhesions: cell's hands They use integrins to grab the ECM and connect to actin inside so the cell can hold on and move |
front 84 What are hemidesmosomes? | back 84 Hemidesmosomes: cell's feet They use integrins to grab laminin outside, and connect to intermediate filaments inside to hold the cell steady |
front 85 What are gap junctions? | back 85 Channels formed by connexons for direct cell-cell communication |