Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Physical Science: Nuclear
front 1 radiation | back 1 energy that comes from a source in the form of waves/rays that you cannot see |
front 2 nucleus | back 2 center of an atom |
front 3 half-life | back 3 the time it takes for half of a radioactive atom undergo radioactive decay |
front 4 radioisotope | back 4 an element that has an unstable nucleus |
front 5 radioactive decay | back 5 emission of energy as electromagnetic waves or as moving subatomic particles |
front 6 Ionizing radiation is | back 6 considered the most harmful |
front 7 During a nuclear reaction, unstable nuclei release ____ of energy | back 7 lots |
front 8 During a nuclear reaction, unstable nuclei try and become _____ stable. | back 8 More |
front 9 When is radiation most dangerous | back 9 when its ingested |
front 10 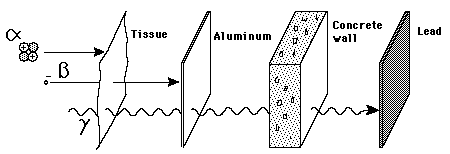 Which ray has the least amount of penetrating power | back 10 alpha |
front 11  Which ray is stopped by lead? | back 11 Gamma |
front 12 40 20Ca --> _____ + 0 -1e | back 12 40 21Sc |
front 13 0 -1e | back 13 beta |
front 14 4 2He | back 14 Alpha |
front 15 1 0n | back 15 neutron |
front 16 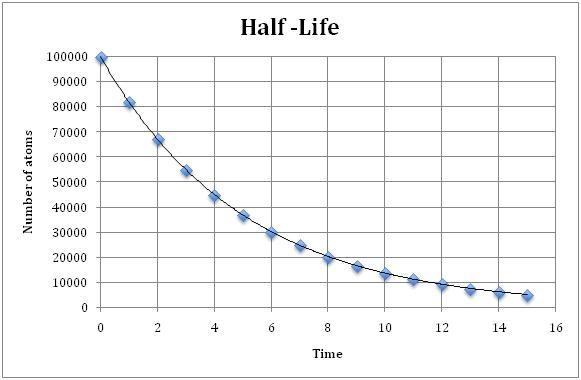 What is the half-life of the graph? | back 16 ~3.4 |
front 17 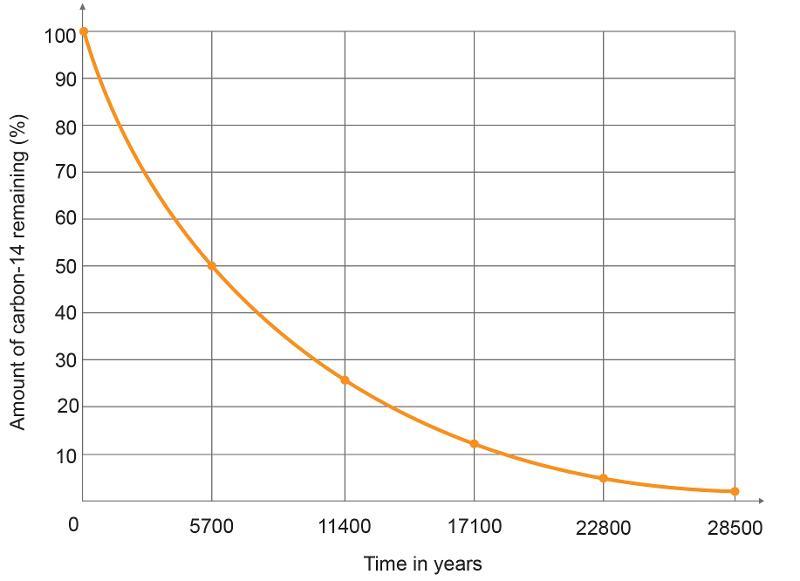 What percentage was left after 11,400 years? | back 17 25% |
front 18 How much time passed after 5% of the substance was left? | back 18 22,800 years |
front 19 True or False. As the number of half-lives increase, the total number of atoms that exist decreases. | back 19 False. They turn into a different substance but still exist. |