Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Results for Boiling Point and Heat of Vaporization SD
front 1 What was your unknown number? | back 1 1 |
front 2 Report the following data: a) Boiling Point (or Range) of the Liquid (in degrees C)? b) Boiling Point of the Liquid (in K), T1? c) Atmospheric Pressure (in mm Hg), P1? | back 2 a) Boiling Point (or Range) of the Liquid (in degrees C) = 75.0 degrees C b) Boiling Point of the Liquid (in K), T1 = 348 K c) Atmospheric Pressure (in mm Hg), P1 = 771.0 mm Hg |
front 3 Determine the vapor pressure of your unknown liquid at 295 K using the Clausius-Clapeyron equation. Show all of you work with units. Next to each appropriate value used, put P1, P2, T1, T2, or R in parentheses. For example, where R is used, 8.314 J/mol/K (R) should be written. If these directions are not followed, a score of 0 will be awarded. | back 3 Vapor pressure of unknown liquid at 295 K = ln (P2/P1) = - (delta H/R) (1/T2 - 1/T1) (P2/P1) = e^ [ - (delta H/R) (1/T2 - 1/T1) ] P2 = P1 e^ [ - (delta H/R) (1/T2 - 1/T1) ] P2 = (771.0 mm Hg) e^ [- ( 38,600 J/mol / 8.314 J/mol/K ) (1/295 K - 1/348 K)] P2 = 70.2 mm Hg |
front 4 Using the video instructions in Course Content of MyCourses, for uploading a graph to MyCourses, do one of the following: a) Upload a picture of your hand-drawn graph. or b) Take a screenshot of your computer generated graph, and upload that .jpg or .png file. Emailed pictures will not be accepted. | back 4 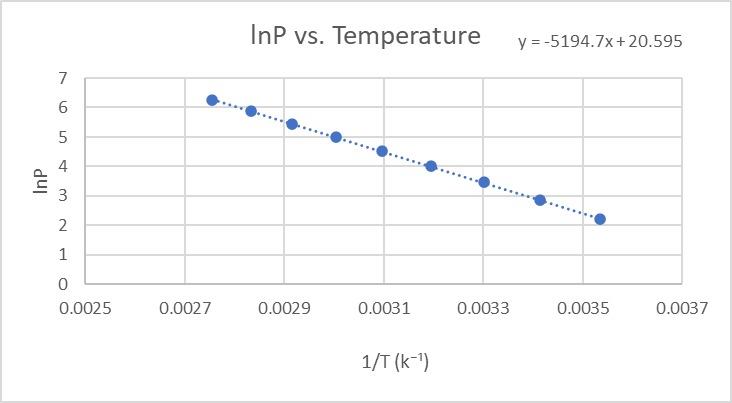 |
front 5 a) Pick two points (NOT data points!) that lie on the line of best fit for your lnP vs 1/T graph, and show the work for calculating the slope, including units. b) Using the equation below, calculate the heat of vaporization of water, in kJ/mole, using 8.314 J/mol K for R. Show all work including units, placing m, ∆H and R next to values in parentheses. For example, where R is used, 8.314 J/mol/K (R) should be written. If these directions are not followed, a score of 0 will be awarded. m = -[delta H/R] | back 5 a) Slope (m) = -5194.7 (we did ours on excel) b) Heat of vaporization of water = m = -[delta H/R] -5194.7 = - delta H / 8.314 J/mol/K -43,189 = - delta H 43,188.7 J/mole = delta H 43,188.7 J/mole x (1 kJ / 1000 J) = delta H 43.189 kJ/mole = delta H |
front 6 Your instructor will give you instructions as what to do with this box. It may be used for bonus points, or for taking away points (for example late submissions) or for error analysis if something went wrong during the lab. If no instructions have been provided to you, please leave this box blank. 5% = 5 | back 6 no data |