Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Postlab for Thin Layer Chromatography SD
front 1 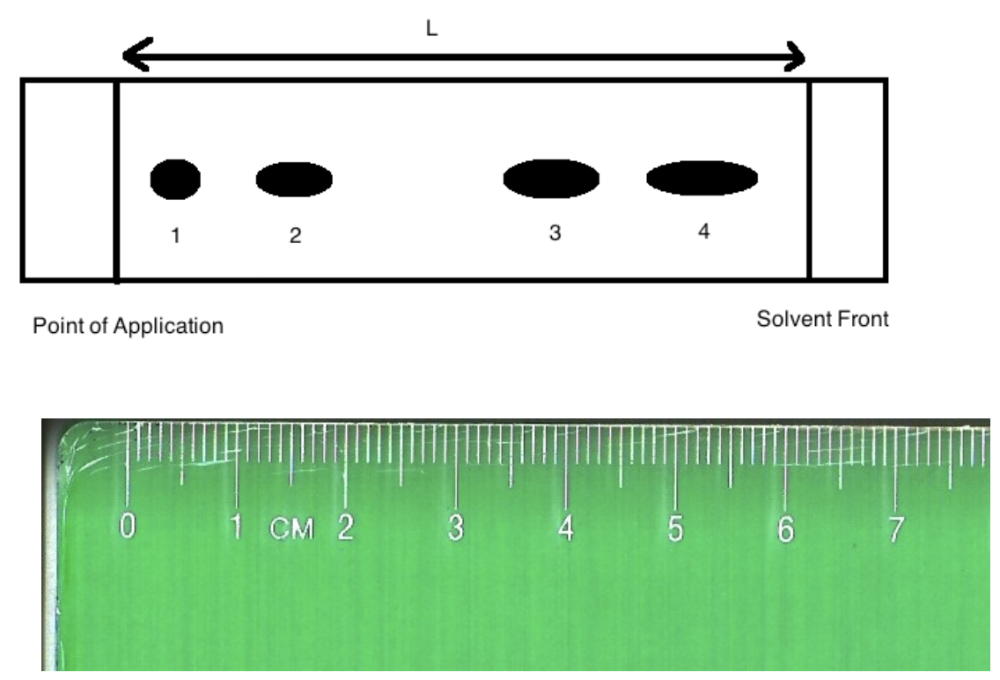 Image: TLC plate with point of application on left with 4 spots. The ruler underneath shows a distance of 6.20 cm from point of application to solvent front and 0.50 cm to spot 1, 1.50 cm to spot 2, 4.00 cm to spot 3 and 5.50 cm to spot 4. End of image. The Rf of substance "3" is:
| back 1 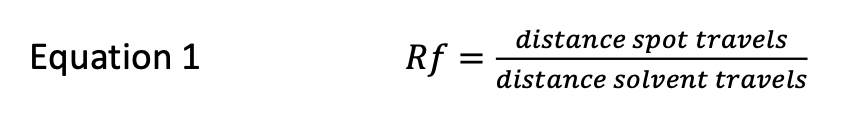 Rf of substance 3 = 4.00 cm / 6.20 cm = 0.65 0.63 |
front 2 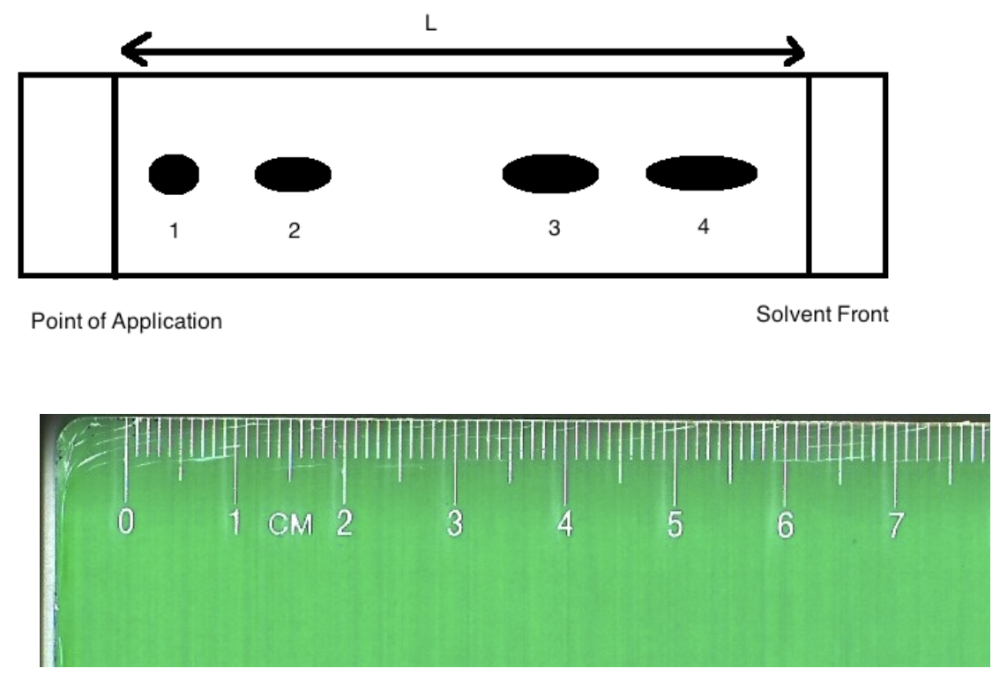 Image: TLC plate with point of application on left with 4 spots. The ruler underneath shows a distance of 6.20 cm from point of application to solvent front and 0.50 cm to spot 1, 1.50 cm to spot 2, 4.00 cm to spot 3 and 5.50 cm to spot 4. End of image. The Rf of substance "4" is:
| back 2 Rf of substance 4 = 5.50 cm / 6.20 cm = 0.89 0.86 |
front 3  The solvent moves 3 cm in about 10 minutes. Why shouldn't the experiment be stopped at that time instead of waiting 75 minutes for the solvent to move 10 cm? | back 3 More time allows for better separation of components in a mixture. |
front 4 In this experiment it takes about 10 microliters of solution to produce a spot 1 cm in diameter. If the C o (NO3)2 solution contains about 6 g C o2+ per liter, how many micrograms of C o2+ ion are there in one spot? 1 microliter = 1E-6 L 1 microgram = 1E-6 g | back 4 (6 g Co2+ / 1 L) x ( 1E-6 g / 1 microgram) x (1L / 1E-6 L) = 6 WRONG Ex. Followed this -> https://www.physicsforums.com/threads/simple-dimensional-analysis-problem-thanks-for-any-help.954241/ Real answer: C= m / V -> m = C x V m = C x V 6 g Co2+ / 1 L x 10 x 10-6 g = 60 x 10-6 g = 60 micrograms |
front 5 Instructor use only. 5% = 2.5 | back 5 no data |
front 6 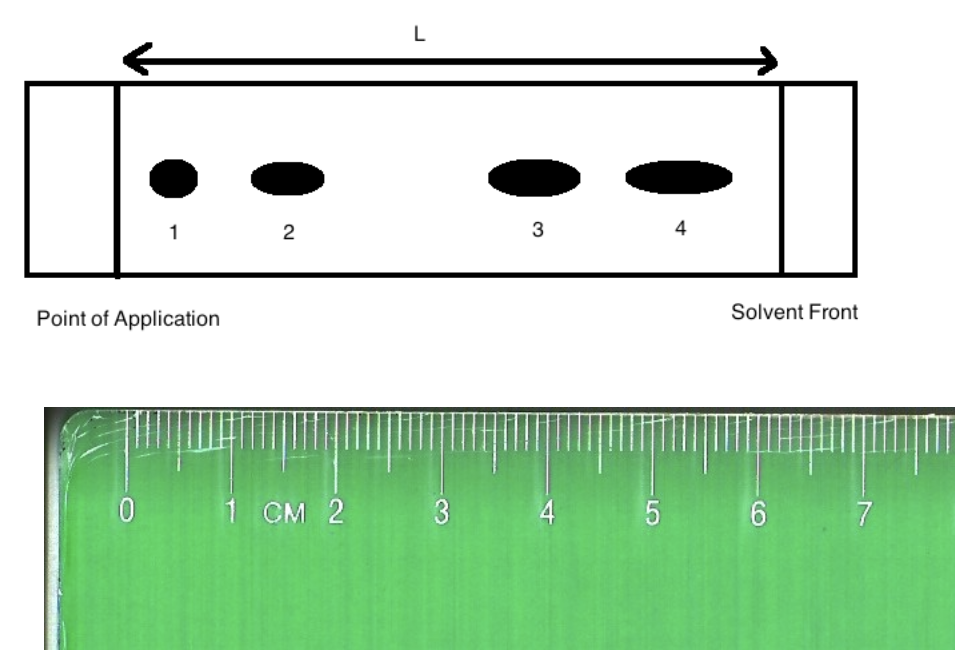 Image: TLC plate with point of application on left with 4 spots. The ruler underneath shows a distance of 6.20 cm from point of application to solvent front and 0.50 cm to spot 1, 1.50 cm to spot 2, 4.00 cm to spot 3 and 5.50 cm to spot 4. End of image. The Rf of substance "3" is:
| back 6 Rf of substance 3 = 4.00 cm / 6.20 cm = 0.65 0.63 |
front 7 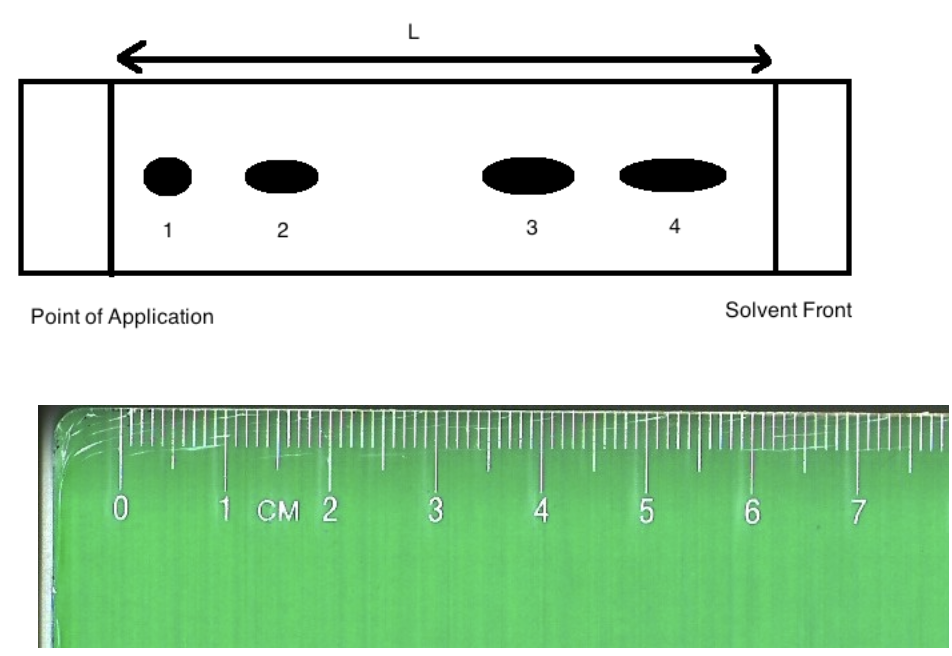 Image: TLC plate with point of application on left with 4 spots. The ruler underneath shows a distance of 6.20 cm from point of application to solvent front and 0.50 cm to spot 1, 1.50 cm to spot 2, 4.00 cm to spot 3 and 5.50 cm to spot 4. End of image. The Rf of substance "4" is:
| back 7 Rf of substance 4 = 5.50 cm / 6.20 cm = 0.89 0.86 |
front 8 The solvent moves 3 cm in about 10 minutes. Why shouldn't the experiment be stopped at that time instead of waiting 75 minutes for the solvent to move 10 cm?
| back 8 More time allows for better separation of components in a mixture. |
front 9 In this experiment it takes about 10 microliters of solution to produce a spot 1 cm in diameter. If the C o (NO3)2 solution contains about 6 g C o2+ per liter, how many micrograms of C o2+ ion are there in one spot? 1 microliter = 1E-6 L 1 microgram = 1E-6 g | back 9 C (concentration) = m (mass) / V (volume) -> m = C x V m = C x V (6 g Co2+ / 1 L) x 10 x 10-6 g = 60 x 10-6 g = 60 micrograms <- ? |
front 10 Instructor use only. 5% = 2.5 | back 10 no data |