Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Results for Thin Layer Chromatography (ONLINE) SD
front 1 What was color of F e3+ before staining?
| back 1 Faint yellow |
front 2 What was color of A g+ before staining?
| back 2 Colorless |
front 3 What was color of B i3+ before staining?
| back 3 Colorless |
front 4 What was color of C o2+ before staining?
| back 4 Faint pink |
front 5 What was color of C u2+ before staining?
| back 5 Faint blue |
front 6 What was color of F e3+ after staining?
| back 6 Blue |
front 7 What was color of A g+ after staining?
| back 7 Greyish or faint yellow |
front 8 What was color of B i3+ after staining?
| back 8 Greyish/Yellow/Orange |
front 9 What was color of C o2+ after staining?
| back 9 Greyish |
front 10 What was color of C u2+ after staining?
| back 10 Burgundy/Brown |
front 11 What were the approximate Rf of the cations on the completed plate? Please use student data. | back 11 Rf Calculations of Cations: Rf of Ag^+ = 0.0009 Rf of Co^2+ = 0.384 Rf of Cu^2+ = 0.512 Rf of Fe^3+ = 0.705 Rf of Bi^3+ = 0.876 Rf Calculations of Unknown Cation Mixture: Rf of Unknown Cation Spot 1 = 0.0009 Rf of Unknown Cation Spot 2 = 0.386 Rf of Unknown Cation Spot 3 = 0.877 |
front 12 Show how one of the Rf values was obtained. (Show work with short verbal descriptions of numbers.) Please use Student Data. | back 12 Rf of Ag^+ = 0.01 cm (distance cation moved) / 11.00 cm (distance solvent moved) = 0.0009 |
front 13 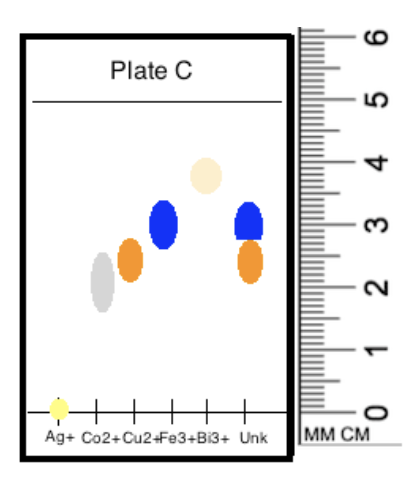 Consider the slide pictured below. What ions are present in the unknown solution? Screen Reader User: Image: Rectangular plate about 6.5 cm high. Horizontal line at 5.5 cm from bottom. Horizontal line 0.5 cm from the bottom marked with A g+, C o2+, C u2+, F e3+, B i3+ and Unk at regular intervals. Vertical ruler starts at 0 cm at horizontal line. A g+ = yellow dot on line. C o 2+ = grey elongated dot at 2 to 3 cm from line. C u2+ = orange dot at 2.5 to 3 cm from line. F e3+ = blue dot 3 to 3.5 cm from line. B i3+ = beige dot at 4 cm from line. Unk = orange dot at 2.5 to 3 cm from line and blue dot 3 to 3.5 cm from line. End of image. | back 13 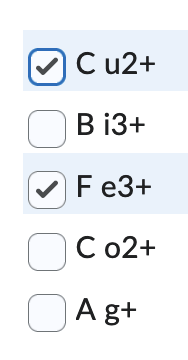 |
front 14 Your instructor will give you instructions as what to do with this box. It may be used for bonus points, or for taking away points (for example late submissions) or for error analysis if something went wrong during the lab. If no instructions have been provided to you, please leave this box blank. 5% = 4 | back 14 no data |