Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Postlab for Spectrophotometric Determination of a Food-Dye SD
front 1  Why were the absorbance measurements recorded at lambda max (504 nm)? | back 1 at lambda max the absorbance is the most sensitive to dye concentration |
front 2  Briefly explain how the following error in the experimental procedure would affect the calculated molarity of Allura Red in the unknown solution. A malfunctioning spectrometer consistently read 10% low on every absorbance reading taken in the experiment. | back 2 Incorrectly Low WRONG Answer: Stay the same |
front 3 An unknown food-dye is analyzed by the method used in this experiment. The absorbance of 5 standard solutions is plotted vs. their concentration (molarity) and a straight line is obtained with the formula: Abs = 3.850x An unknown food-dye solution is diluted and it's absorbance is found to be 0.60. What is the molarity of the diluted unknown, to two significant figures? | back 3 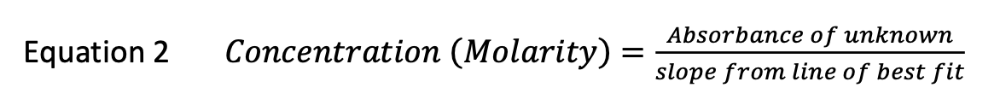 Concentration (Molarity) = 0.60 / 3.850 = 0.16 M |
front 4 Many common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD 50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD 50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD 50 value for humans is the same as for rats. Calculate the number of mg of Allura Red present in an 10 fluid ounce glass of the beverage you used in this lab. Assume that the concentration of Allura Red in the beverage is 0.000044 M. The molar mass of Allura Red is 496.42 grams/mol 1 fl oz = 29.5735 mL | back 4 10 fluid ounce = 10 fl. oz. x 29.5735 mL = 295.735 mL Allura Red = 0.000044 M Number of mg of Allura Red = 295.735 mL x 0.000044 M x 496.42 grams/mol = 6.5 mg |
front 5 Many common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD 50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD 50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD 50 value for humans is the same as for rats. Calculate the number of number of glasses of Allura Red sports drink, each containing 9.4 mg of Allura Red, required to reach the LD 50 of 10,000 mg of Allura Red/kg body weight. Assume the person has a body weight of 130 lbs. 2.205 lbs = 1 kg You may assume two significant figures but do not use scientific notation. Do not include units with your answer. | back 5 130 lbs = 130 lbs x (1 kg / 2.205 lbs) = 58.95692 kg 58.95692 kg x (10,000 mg / 1 kg) = 589569.2 mg 589569.2 mg x (1 sports drink / 9.4 mg of Allura Red) = 62720.12766 -> 62720 |
front 6 Why were the absorbance measurements recorded at lambda max (504 nm)? | back 6 at lambda max the absorbance is the most sensitive to dye concentration |
front 7 Briefly explain how the following error in the experimental procedure would affect the calculated molarity of Allura Red in the unknown solution. A malfunctioning spectrometer consistently read 10% low on every absorbance reading taken in the experiment. | back 7 Stay the same |
front 8 An unknown food-dye is analyzed by the method used in this experiment. The absorbance of 5 standard solutions is plotted vs. their concentration (molarity) and a straight line is obtained with the formula: Abs = 3.200x An unknown food-dye solution is diluted and it's absorbance is found to be 0.50. What is the molarity of the diluted unknown, to two significant figures? | back 8 Concentration (Molarity) = 0.50 / 3.200 = 0.16 M |
front 9 Many common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD 50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD 50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD 50 value for humans is the same as for rats. Calculate the number of mg of Allura Red present in an 11 fluid ounce glass of the beverage you used in this lab. Assume that the concentration of Allura Red in the beverage is 0.000040 M. The molar mass of Allura Red is 496.42 grams/mol 1 fl oz = 29.5735 mL | back 9 11 fluid ounce = 11 fl. oz. x 29.5735 mL = 325.3085 mL Allura Red = 0.000040 M Number of mg of Allura Red = 325.3085 mL x 0.000040 M x 496.42 grams/mol = 6.5 mg |
front 10 Many common materials that we ingest, though quite safe in reasonable quantities, become toxic when taken in very large doses. A measure of toxicity is the LD 50 value (Lethal Dose, 50%). It is the quantity of material, expressed in mg of material per kg of subject-body-weight that, if administered to a population of subjects, would cause 50% of the population to die. The LD 50 value for FD&C Red Dye No. 40 is >10,000 mg/kg in rats. Assume that the LD 50 value for humans is the same as for rats. Calculate the number of number of glasses of Allura Red sports drink, each containing 10.0 mg of Allura Red, required to reach the LD 50 of 10,000 mg of Allura Red/kg body weight. Assume the person has a body weight of 140 lbs. 2.205 lbs = 1 kg You may assume two significant figures but do not use scientific notation. Do not include units with your answer. | back 10 140 lbs = 140 lbs x (1 kg / 2.205 lbs) = 63.492063 kg 63.492063 kg x (10,000 mg / 1 kg) = 634920.63 mg 634920.63 mg x (1 sports drink / 10.0 mg of Allura Red) = 63492.063 -> 63492 |