Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Prelab for Spectrophotometric Determination of a Food-Dye SD
front 1 What food dye will you be testing for? | back 1 Red 40 |
front 2 What color does the food dye you will be testing for mostly absorb? | back 2 green |
front 3 In the formula A = εbc what does the "A" represent? | back 3 absorbance |
front 4 If the Alura Red food-dye stock solution has a molarity of 0.42 M and 2.00 mL of this stock solution is diluted to 100.00 mL, what is the concentration of the diluted solution? Do not use scientific notation. | back 4 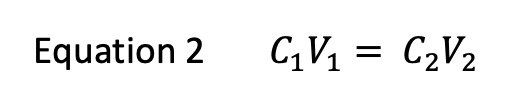 (0.42 M)(2.00 mL) = (C2)(100 mL) C2 = 0.0084 M Ex. where C1 is the concentration (molarity) of the stock Allura Red solution, V1 is the amount of stock solution used, C2 is the concentration of the diluted solution, and V2 is the volume of the diluted solution. |
front 5 An unknown food-dye is analyzed by the method used in this experiment. The absorbance of 5 standard solutions is plotted vs. their concentration (molarity) and a straight line is obtained with the formula: Abs = 2.100x An unknown food-dye solution is diluted and it's absorbance is found to be 0.65. What is the molarity of the diluted unknown, to two significant figures? | back 5 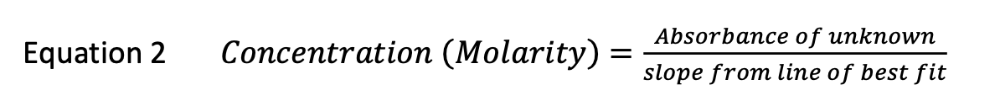 Concentration (Molarity) = 0.65 / 2.100 = 0.31 |