Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Chapter 11
front 1 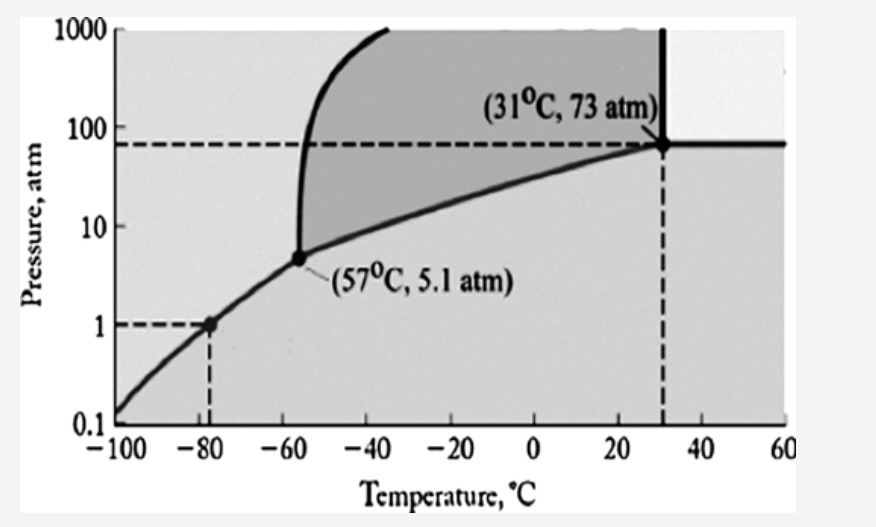 The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature and pressure (22oC, 1 atm)? Answers: gas liquid solid supercritical fluid | back 1 gas |
front 2 For molecules, atoms, or ions with the same mass, which of the following force typically is the strongest intermolecular interaction? Answers: ion dipole hydrogen bonding dipole dipole dispersion ion-induced dipole | back 2 Ion dipole |
front 3 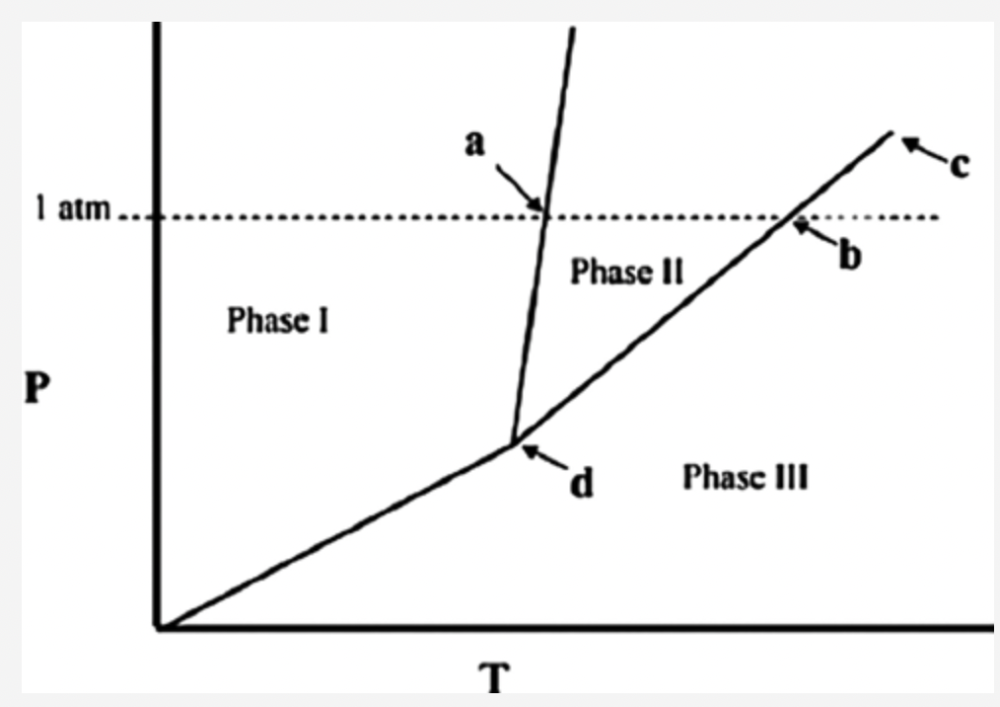 The temperature at point b in the phase diagram below is the ________ Answers: critical point. triple point. transition point. normal freezing point. normal boiling point. | back 3 normal boiling point. |
front 4 Which of the following polar compounds is likely to have the highest boiling point? Answers: CH3OCH3 CH3CH2OH (CH3)2CO H2CO CO | back 4 CH3CH2OH |
front 5 Which molecule has the largest dipole? Answers: methane (CH4) ammonia (NH3) sulfur trioxide (SO3) carbon dioxide (CO2) ethylene (C2H4) | back 5 ammonia (NH3) |
front 6 Which molecule does not exhibit hydrogen bonding? Answers: HF CH3NH2 CH3OH (CH3)3N CH3CH2OH | back 6 (CH3)3N |
front 7 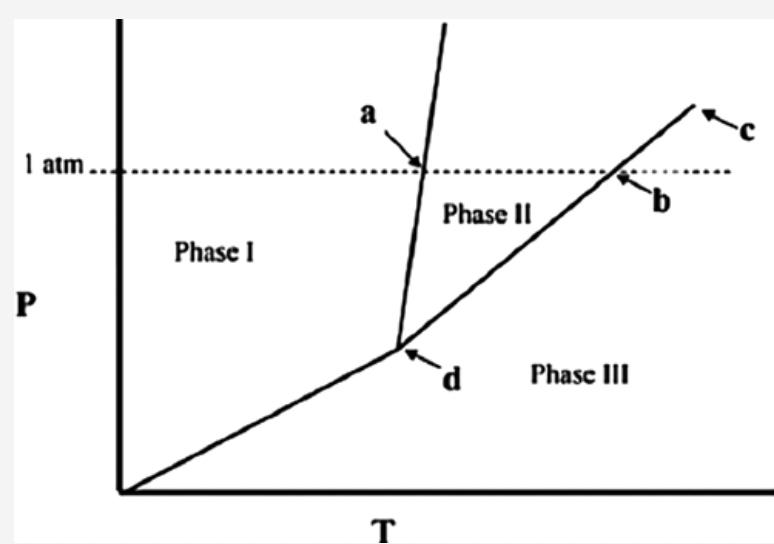 The temperature at point a in the phase diagram below is the ________ Answers: critical point. triple point. transition point. normal freezing point. normal boiling point. | back 7 normal freezing point. |
front 8 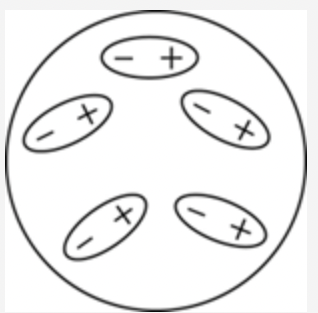 Which of the following diagrams best shows a set of polar molecules interacting through dipole–dipole interactions? | back 8  |
front 9 Based on their boiling points, which of the following compounds has the largest dipole dipole interaction? Answers: propane (231 K) dimethyl ether (248 K) acetonitrile (355 K) methyl chloride (249 K) butane (135 K) | back 9 acetonitrile (355 K) |
front 10 Which of the following compounds is capable of dipole–dipole interactions? Answers: CH4 CO2 H2CO SF6 NH4 + | back 10 H2CO |
front 11 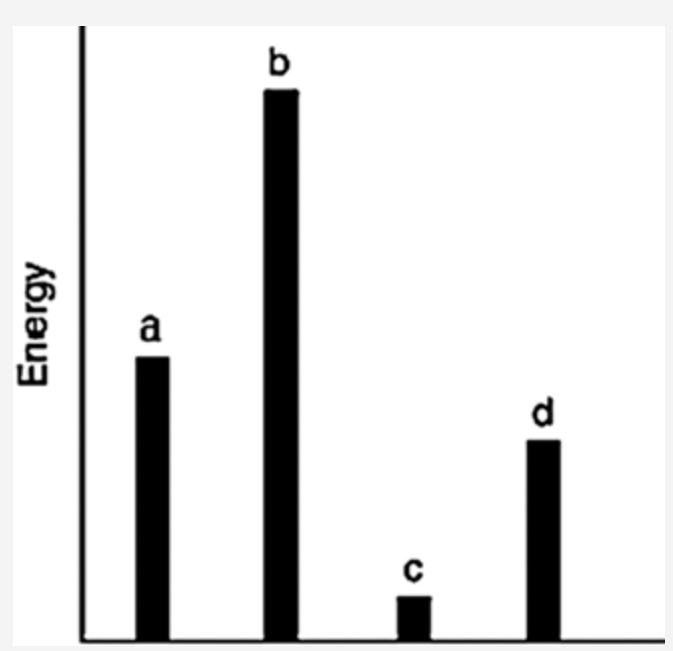 The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the highest boiling point? Answers: a b c d | back 11 b |
front 12 ndicate which of the following nonpolar compounds will have the lowest boiling point. Answers: CCl4 CI4 CF4 CH4 CBr4 | back 12 CH4 |
front 13 Which of the following compounds will not possess dipole dipole interactions between its molecules? Answers: CF4 NH3 H2S CH2F2 CH3Cl | back 13 CF4 |
front 14  The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the lowest boiling point? Answers: a b c d | back 14 c |
front 15 Indicate which of the following pairs of compounds is most likely to be miscible. Answers: H2O and CH3CH2CH2CH3 Br2 and HI HF and CCl4 CCl4 and Br2 CCl4 and NH3 | back 15 CCl4 and Br2 |
front 16 When sodium chloride dissolves in water, how do the water molecules orient around the ions? Answers: Water molecules are randomly oriented around the ions. The hydrogen atoms point toward both the sodium and the chloride. The oxygen atoms point toward both the sodium and the chloride. Around sodium the hydrogen atoms point toward the sodium, and around chloride the oxygen atoms point toward the chloride. Around sodium the oxygen atoms point toward the sodium, and around chloride the hydrogen atoms point toward the chloride. | back 16 Around sodium the oxygen atoms point toward the sodium, and around chloride the hydrogen atoms point toward the chloride. |
front 17 Which statement about a hydrogen bond is not correct? Answers: "Hydrogen bonds involve hydrogen bonded to carbon, nitrogen, oxygen, or fluorine." "In a hydrogen bond, the hydrogen atom on one molecule is attracted to unpaired electrons associated with nitrogen, oxygen, or fluorine on another molecule." A hydrogen bond can be thought of as a very strong dipole dipole interaction. The presence of hydrogen bonds leads to anomalously high boiling points for liquids. Hydrogen bonds stabilize the double helix structure of DNA. | back 17 "Hydrogen bonds involve hydrogen bonded to carbon, nitrogen, oxygen, or fluorine." |
front 18 Which statement about vapor pressure of a pure liquid is not correct? Answers: The vapor pressure is the pressure of the gas at a given temperature in equilibrium with its liquid phase. The temperature at which the vapor pressure equals 760 torr is called the normal boiling point of the substance. The vapor pressure increases with increasing temperature. The vapor pressure increases with increasing surface area of the liquid. Substances with weak intermolecular forces have high vapor pressures at a given temperature. | back 18 The vapor pressure increases with increasing surface area of the liquid. |
front 19 Viscosity is a measure of a substance s ________ Answers: ability to resist changes in its surface area. surface tension. resistance to flow. compressibility. color. | back 19 resistance to flow. |
front 20 At critical point ________________ Answers: all the liquid has evaporated. the densities of the solid and the liquid are the same. the densities of the solid and the gas are the same. the densities of the gas and the liquid are the same. a critical mass has been reached. | back 20 he densities of the gas and the liquid are the same. |
front 21 Which of the following molecules has the highest boiling point? Answers: CH4 SiH4 SnH4 GeH4 H2 | back 21 SnH4 |
front 22 Which statement about vapor pressure of a pure liquid is not correct? Answers: The vapor pressure is the pressure of the gas at a given temperature in equilibrium with its liquid phase. The temperature at which the vapor pressure equals 760 torr is called the normal boiling point of the substance. The vapor pressure increases with increasing temperature. The vapor pressure increases with increasing surface area of the liquid. Substances with weak intermolecular forces have high vapor pressures at a given temperature. | back 22 The vapor pressure increases with increasing surface area of the liquid. |
front 23  he temperature at point b in the phase diagram below is the ________ Answers: critical point. triple point. transition point. normal freezing point. normal boiling point. | back 23 normal boiling point. |
front 24 The vapor pressure of a liquid will increase if ________ Answers: the temperature is increased. the volume available to the gas phase is increased. the volume of the liquid phase is increased. the liquid is stirred. the liquid is put in a container with a larger surface area. | back 24 the temperature is increased. |
front 25  The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature and pressure (22oC, 1 atm)? Answers: gas liquid solid supercritical fluid | back 25 gas |
front 26 Which of the following molecules will have the least interaction with a Li+ ion? Answers: water (H2O) ethanol (CH3CH2OH) chloroform (CHCl3) ammonia (NH3) pentane (C5H12) | back 26 pentane (C5H12) |
front 27 Of all the noble gases, ________ has the weakest intermolecular force and hence the lowest boiling point. Answers: He Ne Ar Kr Xe | back 27 He |
front 28 Which is the dominant interaction between acetone molecules, (CH3)2CO? Answers: ion ion ion dipole dipole dipole hydrogen bonding dispersion or London forces | back 28 dipole, dipole |
front 29 Normal boiling points of branched alkane hydrocarbons generally are lower than for straight-chain hydrocarbons of the same molar mass because the straight-chain hydrocarbons ________ Answers: have stronger dipole dipole interactions. have many more electrons. have more C H bonds. have a larger surface area and come in closer contact with each other. react with each other to form long chains. | back 29 have a larger surface area and come in closer contact with each other. |
front 30 Which of the following non-polar molecules will have the highest boiling point? Answers: CO2 C6H6 (benzene) C6F6 (hexafluorobenzene) C2H2 CF4 | back 30 C6F6 (hexafluorobenzene) |
front 31 Which of the following molecules has the highest boiling point? Answers: CH4 SiH4 SnH4 GeH4 H2 | back 31 SnH4 |
front 32 Which is the dominant interaction between oxygen and nitrogen molecules in air? Answers: ion ion ion dipole dipole dipole hydrogen bonding dispersion or London forces | back 32 dispersion or London forces |
front 33 Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the combustion properties of isooctane. Gasoline usually contains an isomer of isooctane called tetramethylbutane (C8H18), which has an enthalpy of vaporization of 43.3 kJ/mol and a boiling point of 106.5°C. Determine the vapor pressure of tetramethylbutane on a very hot summer day when the temperature is 38°C. Answers: 80.0 torr 36.7 torr 67.8 torr 47.9 torr 89.3 torr | back 33 36.7 torr |
front 34 Which of the following compounds will not possess dipole dipole interactions between its molecules? Answers: CF4 NH3 H2S CH2F2 CH3Cl | back 34 CF4 |
front 35 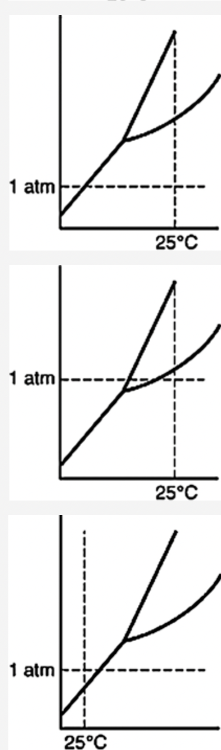 Which of the following substances is a solid at 25°C and 1 atm? | back 35 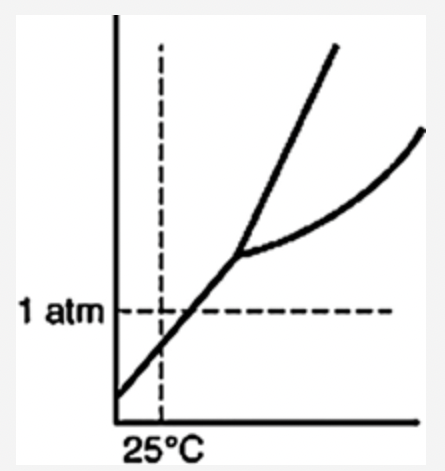 |
front 36 The smell of fresh-cut pine is due in part to a cyclic alkene called pinene. A graph of the natural logarithm of the vapor pressure of pinene vs. 1/temperature produces a straight line with a slope of -4936.37 K. What is the enthalpy of vaporization of pinene? Answers: +397 kJ/mol 39.7 kJ/mol +39.7 kJ/mol - 41.0 kJ/mol +41.0 kJ/mol | back 36 +41.0 kJ/mol |
front 37 Which of the following compounds is capable of hydrogen bonding?Which of the following compounds is capable of hydrogen bonding?Answers: CH3OCH3 CH3COCH3 CH3CH2OH H2CO CH3F | back 37 CH3CH2OH |
front 38 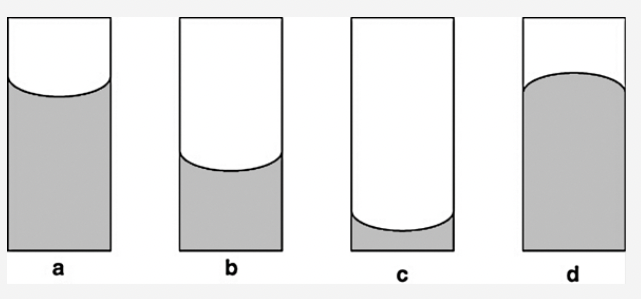 In which of the following diagrams are the cohesive interactions between molecules in the liquid greater than the adhesive forces between the liquid and the walls of the tube? Answers: a b c d | back 38 d |
front 39 Which of the following polar compounds is likely to have the highest boiling point? Answers: CH3OCH3 CH3CH2OH (CH3)2CO H2CO CO | back 39 CH3CH2OH |
front 40 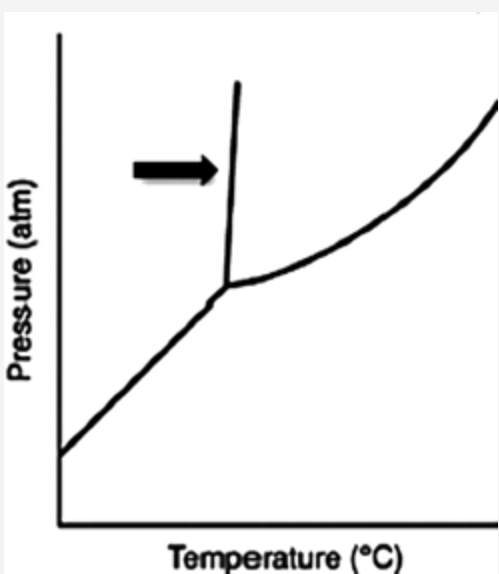 What does the line indicated by the arrow in the following phase diagram represent? Answers: solid–liquid boundary solid–gas boundary liquid–gas boundary triple point solid–solid boundary | back 40 solid–liquid boundary |
front 41 Based on their boiling points, which of the following compounds has the largest dipole dipole interaction? Answers: propane (231 K) dimethyl ether (248 K) acetonitrile (355 K) methyl chloride (249 K) butane (135 K) | back 41 acetonitrile (355 K) |
front 42 Which type of intermolecular interaction exists for all compounds?Answers: ion ion dipole dipole dispersion hydrogen bonding dipole-induced dipole | back 42 dispersion |
front 43 When sodium chloride dissolves in water, how do the water molecules orient around the ions? Answers: Water molecules are randomly oriented around the ions. The hydrogen atoms point toward both the sodium and the chloride. The oxygen atoms point toward both the sodium and the chloride. Around sodium the hydrogen atoms point toward the sodium, and around chloride the oxygen atoms point toward the chloride. Around sodium the oxygen atoms point toward the sodium, and around chloride the hydrogen atoms point toward the chloride. | back 43 Around sodium the oxygen atoms point toward the sodium, and around chloride the hydrogen atoms point toward the chloride. |
front 44 Of all the noble gases, ________ has the weakest intermolecular force and hence the lowest boiling point. Answers: He Ne Ar Kr Xe | back 44 He |
front 45  Which of the substances a–d in the following figure has the weakest intermolecular forces? a b c d | back 45 c |
front 46 Which intermolecular force is caused by an instantaneous dipole generated by close contact with other atoms or molecules? Answers: ion ion forces ion dipole forces hydrogen bonding dipole dipole forces dispersion forces | back 46 dispersion forces |
front 47  The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the lowest boiling point? a b c d | back 47 c |
front 48  Which of the following diagrams best shows a set of polar molecules interacting through dipole–dipole interactions? | back 48  |
front 49  What does the line indicated by the arrow in the following phase diagram represent? Answers: solid–liquid boundary solid–gas boundary liquid–gas boundary triple point solid–solid boundary | back 49 solid–liquid boundary |
front 50 Which alkane compound has the lowest boiling point? Answers: C4H10 C5H12 C6H14 C7H16 C8H18 | back 50 C4H10 |