Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Central Science: Chapter 14
front 1 A burning splint will burn more vigorously in pure oxygen than in air
because ________. | back 1 A |
front 2 Of the following, all are valid units for a reaction rate except
________. | back 2 A |
front 3 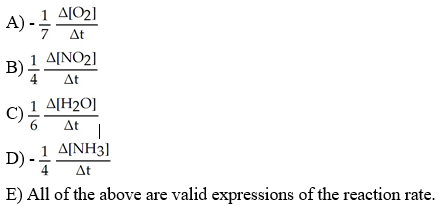 Which one of the following is not a valid expression for the rate of the reaction below? 4NH3 + 7O2 → 4NO2 + 6H2O | back 3 E |
front 4 The rate law of a reaction is rate = k[D][X]. The units of the rate
constant are ________. | back 4 B |
front 5 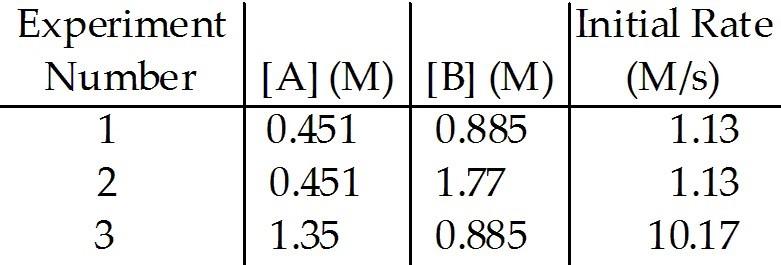 The data in the table below were obtained for the reaction: A + B → C 5) The rate law for this reaction is rate = ________. | back 5 E |
front 6  The data in the table below were obtained for the reaction: A + B → C The magnitude of the rate constant is ________. | back 6 A |
front 7 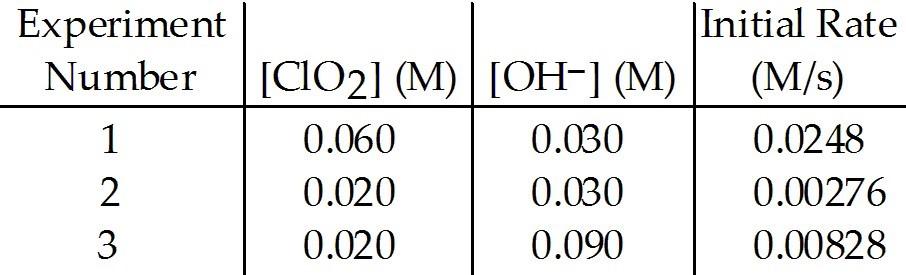 The data in the table below were obtained for the reaction: 2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) 7) What is the order of the reaction with respect to ClO2? | back 7 C |
front 8  The data in the table below were obtained for the reaction: 2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) What is the order of the reaction with respect to OH-? | back 8 B |
front 9  The data in the table below were obtained for the reaction: 2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) Which of the following is correct regarding the overall order of
the reaction? | back 9 C |
front 10 The data in the table below were obtained for the reaction: 2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) What is the magnitude of the rate constant for the
reaction? | back 10 C |
front 11 Under constant conditions, the half-life of a first-order reaction
________. | back 11 E |
front 12 The reaction 2NO2 → 2NO + O2 follows second-order kinetics. At 300 °C, [NO2] drops from 0.0100
M to 0.00650 M in 100.0 s. The rate constant for the reaction is
________ M-1s-1. | back 12 E |
front 13 The reaction CH3-N≡C → CH3-C≡N is a first-order reaction. At 230.3 °C, k = 6.29 x
10-4S-1. If [CH3-N≡C] is 1.00 × 10-3
initially, [CH3-N≡C] is ________ M after 1.000 x
103s. | back 13 A |
front 14 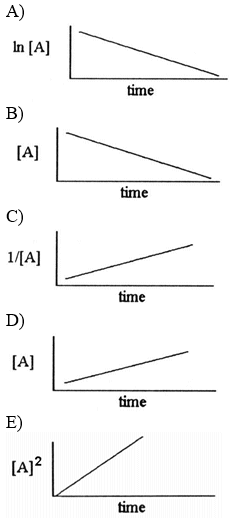 Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]? | back 14 C |
front 15 The following reaction is second order in [A] and the rate constant is 0.025 M-1s-1: A → B The concentration of A was 0.65 M at 33 s. The initial
concentration of A was ________ M. | back 15 D |
front 16 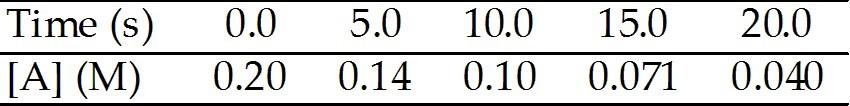 The reaction A → B is first order in [A]. Consider the following data. 16) What is the rate constant (s-1) for this
reaction? | back 16 E |
front 17 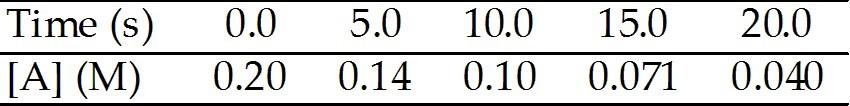 The reaction A → B is first order in [A]. Consider the following data. What is the concentration (M) of [A] after 80.0 s? | back 17 D |
front 18 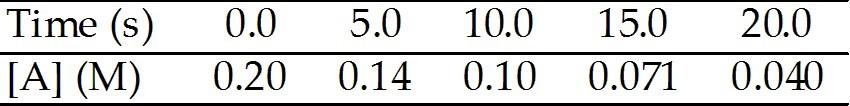 The reaction A → B is first order in [A]. Consider the following data. The rate constant of a first-order process that has a half-life of
3.50 min is ________ s-1. | back 18 E |
front 19  The reaction A (aq) → B (aq) is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: The rate constant for this reaction is ________ s-1.
| back 19 D |
front 20 One difference between first- and second-order reactions is that
________. | back 20 A |
front 21 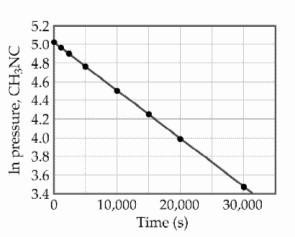 At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN): CH3NC (g) → CH3CN (g) The reaction is first order in methylisonitrile. The attached graph shows data for the reaction obtained at 198.9 °C. What is the rate constant (s-1) for the reaction? | back 21 B |
front 22 The decomposition of [A] in solution at 55 °C proceeds via first order: A (aq) → B (aq) What is the rate law for the reaction? | back 22 B |
front 23 As the temperature of a reaction is increased, the rate of the
reaction increases because the ________. | back 23 B |
front 24 The rate of a reaction depends on ________. | back 24 D |
front 25 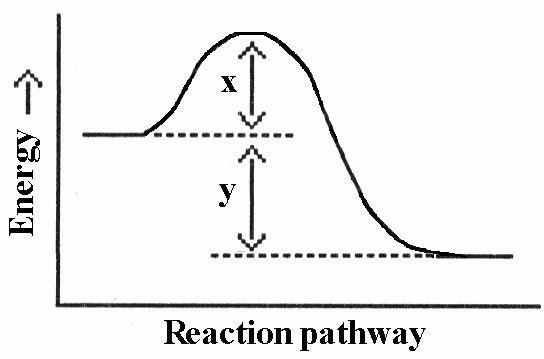 Which energy difference in the energy profile below corresponds to the activation energy for the forward reaction? A) x | back 25 A |
front 26 In the energy profile of a reaction, the species that exists at the
maximum on the curve is called the ________. | back 26 B |
front 27 In the Arrhenius equation, k = Ae-Ea/RT ________ is the frequency factor. | back 27 B |
front 28 In general, as temperature goes up, reaction rate ________. | back 28 C |
front 29 In general, as activation energy increases, reaction rate
________. | back 29 D |
front 30 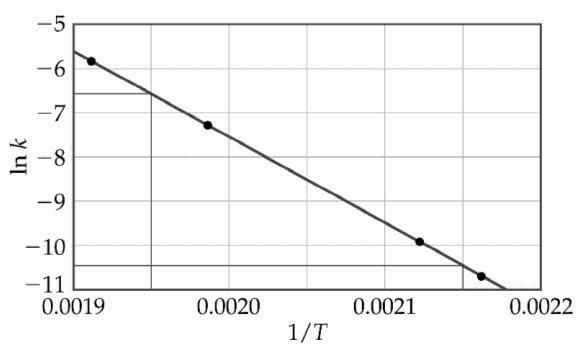 The decomposition of [A] in solution at 80 °C proceeds via the following reaction: A (aq) → B (aq) The dependence of the rate constant on temperature is studied and the graph below is prepared from the results. What is the energy of activation (kJ/mol) for this
reaction? | back 30 C |
front 31 The mechanism for formation of the product X is: A + B → C + D (slow) The intermediate reactant in the reaction is ________. | back 31 D |
front 32 For the elementary reaction NO3 + CO → NO2 + CO2 the molecularity of the reaction is ________, and the rate law is
| back 32 A |
front 33 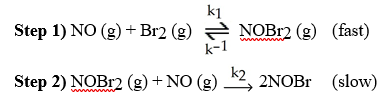 A possible mechanism for the overall reaction Br2 (g) + 2NO (g) → 2NOBr (g) is [see image] What is the rate determining step for this reaction? | back 33 A |
front 34 Which of the following is true? | back 34 A |
front 35 Of the following, ________ will lower the activation energy for a
reaction. | back 35 C |
front 36 The rate law of the overall reaction A + B → C is rate = k[A]2. Which of the following will not
increase the rate of the reaction? | back 36 B |
front 37 A catalyst can ________ the rate of a reaction by providing an
alternative pathway with a ________ activation energy | back 37 B |
front 38 The primary source of the specificity of enzymes is ________. | back 38 E |
front 39 ________ are used in automotive catalytic converters. | back 39 A |
front 40 The enzyme ________ converts nitrogen into ammonia. | back 40 E |
front 41 Fe and Mo are transition metals found in the cofactor active site of
________. | back 41 B |
front 42 Which of the following statements describes why nitrogen fixation is
a difficult process? | back 42 B |
front 43 Consider the following reaction: 3A → 2B The average rate of appearance of B is given by Δ[B]/Δt. Comparing
the rate of appearance of B and the rate of disappearance of A, we get
Δ[B]/Δt = ________ x (-Δ[A]/Δt). | back 43 B |
front 44 Which substance in the reaction below either appears or disappears the fastest? 4NH3 + 7O2 → 4NO2 + 6H2O A) NH3 | back 44 B |
front 45 Consider the following reaction: A → B The average rate of appearance of B is given by Δ[B]Δt. Comparing
the rate of appearance of B and the rate of disappearance of A, we get
Δ[B]/Δt = ________ x (-Δ[A]/Δt). | back 45 A |
front 46  A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 10 s and 20 s is
________ mol/s. | back 46 A |
front 47  A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 20 s and 40 s is
________ mol/s. | back 47 B |
front 48  A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of appearance of B between 20 s and 30 s is
________ mol/s. | back 48 A |
front 49  A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: The average rate disappearance of A between 20 s and 30 s is
________ mol/s. | back 49 C |
front 50  A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 40. s? | back 50 B |
front 51  A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 30 s? | back 51 E |
front 52 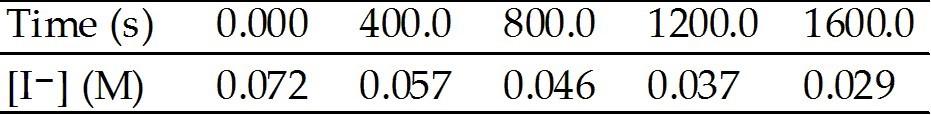 The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction: S2O82- (aq) + 3I- → 2SO42- (aq) + I3- (aq) An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. The average rate of disappearance of I- between 400.0 s and 800.0 s
is ________ M/s. | back 52 A |
front 53  The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction: S2O82- (aq) + 3I- → 2SO42- (aq) + I3- (aq) An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. What is the average rate of disappearance (M/s) of I- in the
initial 800.0 s? | back 53 C |
front 54  The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction: S2O82- (aq) + 3I- → 2SO42- (aq) + I3- (aq) An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. What is the average rate of disappearance (M/s) of I- between 800.0
s and 1200.0 s? | back 54 D |
front 55  The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction: S2O82- (aq) + 3I- → 2SO42- (aq) + I3- (aq) An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. What is the concentration (M) of S2O82- remaining at
1200 s? | back 55 B |
front 56  The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction: S2O82- (aq) + 3I- → 2SO42- (aq) + I3- (aq) An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. The concentration of S2O82- remaining at 800 s is
________ M. | back 56 E |
front 57  The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction: S2O82- (aq) + 3I- → 2SO42- (aq) + I3- (aq) An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. What is the concentration (M) of S2O82- remaining at
T=0? | back 57 D |
front 58 At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g) → 4NO2 (g) + O2 (g) When the rate of formation of NO2 is 5.5 × 10-4 M/s, the
rate of decomposition of N2O5 is ________ M/s. | back 58 D |
front 59 At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN): CH3NC (g) → CH3CN (g) At the start of the experiment, there are 0.200 mol of reactant
(CH3NC) and 0 mol of product (CH3CN) in the reaction vessel. After 25
min of reaction, 0.108 mol of reactant (CH3NC) remain. The average
rate of decomposition of methyl isonitrile, CH3NC, in this 25 min
period is ________ mol/min. | back 59 A |
front 60 A reaction was found to be second order in carbon monoxide
concentration. The rate of the reaction ________ if the [CO] is
doubled, with everything else kept the same. | back 60 D |
front 61 ) If the rate law for the reaction 2A + 3B → products is first order in A and second order in B, then the rate law is
| back 61 C |
front 62 If the rate law for the reaction A + 3B → C + 2D is zero order in A and first order in B, then the rate law is
| back 62 C |
front 63 The overall order of a reaction is 1. The units of the rate constant
for the reaction are ________. | back 63 C |
front 64 The overall order of a reaction is 2. The units of the rate constant
for the reaction are ________. | back 64 B |
front 65 It was determined experimentally that the reaction rate doubled when the concentration of B was doubled in the following reaction: A + B → C The reaction is ________ order in B. | back 65 B |
front 66 It was determined experimentally that the reaction rate remained the same when the concentration of A was doubled in the following reaction: A + B → C The reaction is ________ order in A. | back 66 A |
front 67 A reaction was found to be third order in A. Increasing the
concentration of A by a factor of 3 will cause the reaction rate to
________. | back 67 B |
front 68 A reaction was found to be zero order in A. Increasing the
concentration of A by a factor of 3 will cause the reaction rate to
________. | back 68 A |
front 69 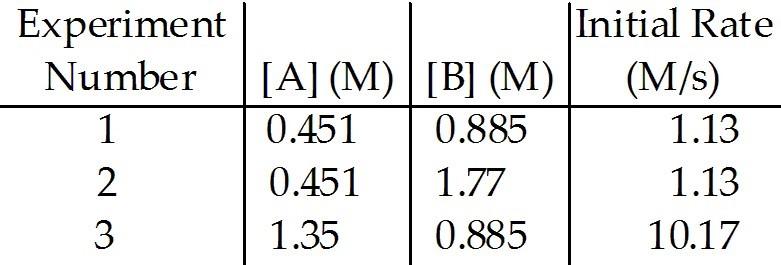 The data in the table below were obtained for the reaction: A + B → C What is the order of the reaction in A? | back 69 B |
front 70  The data in the table below were obtained for the reaction: A + B → C What is the order of the reaction in B? | back 70 E |
front 71  The data in the table below were obtained for the reaction: A + B → C What is the overall order of the reaction? | back 71 B |
front 72 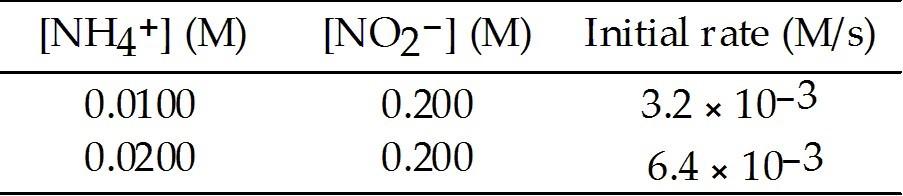 The following reaction occurs in aqueous solution: NH4+ (aq) + NO2- (aq) → N2 (g) + 2H2O (l) The data below is obtained at 25 °C. The order of the reaction in NH4+ is ________. | back 72 D |
front 73 For a zero-order reaction, a plot of ________ versus ________ is
linear. | back 73 B |
front 74 The half-life of a first-order reaction is 13 min. If the initial
concentration of reactant is 0.085 M, it takes ________ min for it to
decrease to 0.055 M. | back 74 A |
front 75 The half-life of a first-order reaction is 13 min. If the initial
concentration of reactant is 0.13 M, it takes ________ min for it to
decrease to 0.085 M. | back 75 C |
front 76 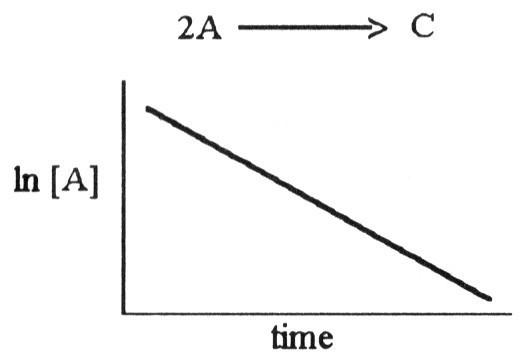 The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to ________. | back 76 D |
front 77 The reaction below is first order in [H2O2]: 2H2O2 (l) → 2H2O (l) + O2 (g) A solution originally at 0.600 M H2O2 is found to be 0.075 M after
54 min. The half-life for this reaction is ________ min. | back 77 B |
front 78 A second-order reaction has a half-life of 18 s when the initial
concentration of reactant is 0.71 M. The rate constant for this
reaction is ________ M-1s-1. | back 78 A |
front 79 A second-order reaction has a half-life of 12 s when the initial
concentration of reactant is 0.98 M. The rate constant for this
reaction is ________ M-1s-1. | back 79 C |
front 80 Of the following, only ________ is a valid unit for reaction
rate. | back 80 A |
front 81 The overall reaction below is ________ and the elementary reaction is considered ________. AB → A + B A) unimolecular, rare | back 81 C |
front 82 Of the units below, ________ are appropriate for a third-order
reaction rate constant. | back 82 A |
front 83 The rate law for a reaction is rate = k[A][B] Which one of the following statements is false? | back 83 A |
front 84 Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2NO2 → 2NO + O2 In a particular experiment at 300 °C, [N ] drops from 0.0143 to
0.00701 M in 261 s. The rate of disappearance of NO2 for this period
is ________ M/s. | back 84 A |
front 85 At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g) → 4NO2 (g) + O2 (g) When the rate of formation of O2 is 3.6 × 10-3 M/s, the
rate of decomposition of N2O5 is | back 85 A |
front 86 The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g) is 0.190 M s-1 at 150 °C. The rate of appearance of Br2
is ________ M s-1. | back 86 B |
front 87 The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g) is 0.140 M s-1 at 150 °C. The rate of reaction is
________ M s-1. | back 87 B |
front 88 During the combustion of ethylene gas, C2H4, carbon dioxide is
produced and the rate of disappearance of O2 is 0.13 M s-1.
What is the rate of appearance (M s-1) of CO2? | back 88 C |
front 89 The combustion of ethylene proceeds by the reaction C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (g) When the rate of disappearance of O2 is 0.13 M s-1, the
rate of disappearance of C2H4 is ________ M s-1
| back 89 B |
front 90 Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2NO2 (g) → 2NO (g) + O2 (g) In a particular experiment at 300 °C, [NO2] drops from 0.0100 to
0.00800 M in 100 s. The rate of appearance of O2 for this period is
________ M/s. | back 90 A |
front 91 At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN): CH3NC (g) → CH3CN (g) At the start of an experiment, there are 0.200 mol of reactant and 0
mol of product in the reaction vessel. After 25 min, 0.121 mol of
reactant (CH3NC) remain. There are ________ mol of product (CH3CN) in
the reaction vessel. | back 91 E |
front 92 A compound decomposes by a first-order process. If 13% of the
compound decomposes in 60 minutes, the half-life of the compound is
________ min. | back 92 A |
front 93 What value is represented by the y-intercept of the line drawn from
plotting [A] vs. time in a linear graphical representation of a
zero-order reaction? | back 93 C |
front 94 The rate constant for a particular zero-order reaction is 0.075 M
s-1. If the initial concentration of reactant is 0.537 M it
takes ________ s for the concentration to decrease to 0.100 M. | back 94 A |
front 95 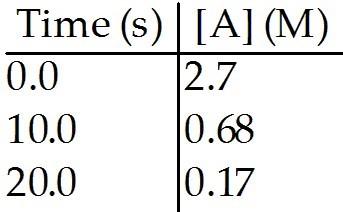 The reaction A → B is first order in [A]. Consider the following data. The rate constant for this reaction is ________
s-1. | back 95 A |
front 96 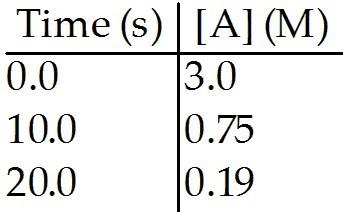 The reaction A → B is first order in [A]. Consider the following data. What is the half-life, in seconds, of this reaction? | back 96 A |
front 97 What is the rate constant ( s-1) of a first-order process
that has a half-life of 550 s? | back 97 D |
front 98 The following reaction is second order in [A] and the rate constant is 0.039 M-1s-1: A → B What is the initial concentration (M) of A if at 28 s the
concentration of A was 0.26 M? | back 98 C |
front 99 What is the half-life of an unknown compound if 17.0% of the compound
decomposes in 150 minutes via a first-order process? | back 99 E |
front 100 The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g) is first order in CH3NC. The rate constant for the reaction is 9.45
× 10-5 s-1 at 478 K. The half-life of the
reaction when the initial [CH3NC] is 0.030 M is ________ s. | back 100 D |
front 101 The elementary reaction 2NO2 (g) → 2NO (g) + O2 (g) is second order in NO2 and the rate constant at 660 K is 5.23
M-1s-1. The reaction half-life at this
temperature when [NO2]0 = 0.45 M is ________ s. | back 101 E |
front 102 The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g) is first order in CH3NC. The half-life of the reaction is 2.70 ×
104 s at 463 K. The rate constant is ________
s-1 when the initial [CH3NC] is 0.030 M. | back 102 C |
front 103 The decomposition of in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln) The reaction is first order and has a rate constant of 4.82 ×
10-3 s-1 at 64 °C. If the reaction is initiated
with 0.072 mol in a 1.00-L vessel, how many moles remain after 151
s? | back 103 C |
front 104 SO2Cl2 decomposes in the gas phase by the reaction SO2Cl2 (g) → SO2 (g) + Cl2 (g) The reaction is first order in SO2Cl2 and the rate constant is 3.0 ×
10-6 s-1 at 600 K. A vessel is charged with 3.6
atm of SO2Cl2 at 600 K. The partial pressure of SO2Cl2 at 3.0 x
105 s is ________ atm. | back 104 C |
front 105 The rate constant for a particular second-order reaction is 0.47
M-1s-1. If the initial concentration of reactant
is 0.25 mol/L, it takes ________ s for the concentration to decrease
to 0.20 mol/L. | back 105 A |
front 106 The reaction 2NOBr (g) → 2 NO (g) + Br2 (g) is a second-order reaction with a rate constant of 0.80 M-1s-1 at 11
°C. If the initial concentration of NOBr is 0.0440 M, the
concentration of NOBr after 6.0 seconds is ________. | back 106 C |
front 107 A first-order reaction has a rate constant of 0.33 min-1.
It takes ________ min for the reactant concentration to decrease from
0.13 M to 0.066 M. | back 107 E |
front 108 The initial concentration of reactant in a first-order reaction is
0.27 M and has a rate constant of 0.75 s-1. What is the
concentration (mol/L) of reactant after 0.75 s? | back 108 D |
front 109 The rate constant for a second-order reaction is 0.13
M-1s-1. If the initial concentration of reactant
is 0.26 mol/L, it takes ________ s for the concentration to decrease
to 0.07 mol/L. | back 109 D |
front 110 At elevated temperatures, nitrogen dioxide decomposes to nitrogen oxide and oxygen: NO2 (g) → NO (g) + 1/2 O2 (g) | back 110 E |
front 111 A particular first-order reaction has a rate constant of 1.35 ×
102 s-1 at 25.0 °C. What is the magnitude of k
at 650 °C if Ea = 55.5 kJ/mol? | back 111 A |
front 112 What is the magnitude of k for a first-order reaction at 75.0 °C if
Ea = 95.1 kJ/mol and rate constant is 1.35 × 102
s-1 at 25.0 °C? | back 112 A |
front 113 Define Beer's Law. | back 113 the direct relationship of absorbed light to the concentration of the substance absorbing the light |
front 114 Write the rate law for the reaction: aA → bB + cC. | back 114 rate = k[A]m |
front 115 If a rate law is first order (reactant), doubling the reactant ________ the reaction rate. | back 115 doubles |
front 116 The Earth's ozone layer is located in the ________. | back 116 stratosphere |
front 117 According to the collision model, reaction rates are affected by reactant ________ and ________. | back 117 concentration, temperature |
front 118 Define activation energy. | back 118 the minimum energy required to initiate a chemical reaction |
front 119 The rate of a chemical reaction should ________ as the temperature rises. | back 119 increase |
front 120 The rate-determining step is the ________ elementary step. | back 120 slowest |
front 121 A unimolecular elementary reaction involves ________ reactant molecule(s). | back 121 one |
front 122 Define a homogeneous catalyst. | back 122 a catalyst that is present in the same phase as the reacting molecules |
front 123 Define a heterogeneous catalyst. | back 123 a catalyst present in a different phase from the reacting molecules |
front 124 Biological catalysts are referred to as ________. | back 124 enzymes |
front 125 A ________ is a substance that speeds up a chemical reaction without itself being consumed in the reaction. | back 125 catalyst |
front 126 Rates of reaction can be positive or negative. | back 126 false |
front 127 The instantaneous rate of a reaction can be read directly from the graph of molarity versus time at any point on the graph. | back 127 false |
front 128 The overall reaction order is the sum of the orders of each reactant in the rate law. | back 128 true |
front 129 Units of the rate constant of a reaction are independent of the overall reaction order. | back 129 false |
front 130 The concentration of reactants or products at any time during the reaction can be calculated from the integrated rate law. | back 130 true |
front 131 The rate of a second order reaction can depend on the concentrations of more than one reactant. | back 131 true |
front 132 The half-life for a first order rate law depends on the starting concentration. | back 132 false |
front 133 The rate limiting step in a reaction is the slowest step in the reaction sequence. | back 133 true |
front 134 Heterogeneous catalysts have different phases from reactants. | back 134 true |