Instructions for Side by Side Printing
- Print the notecards
- Fold each page in half along the solid vertical line
- Cut out the notecards by cutting along each horizontal dotted line
- Optional: Glue, tape or staple the ends of each notecard together
Kidney 3, Acid-base balance In class Questions
front 1 T/F: Equivalents are the milliliters of a substance needed to balance 1 mole of an opposite, monovalent charge. | back 1  F Equivalents are the MOLES of a substance needed to balance 1 mole of an opposite, monovalent charge. |
front 2 T/F: Strong base slowly dissociates in water | back 2 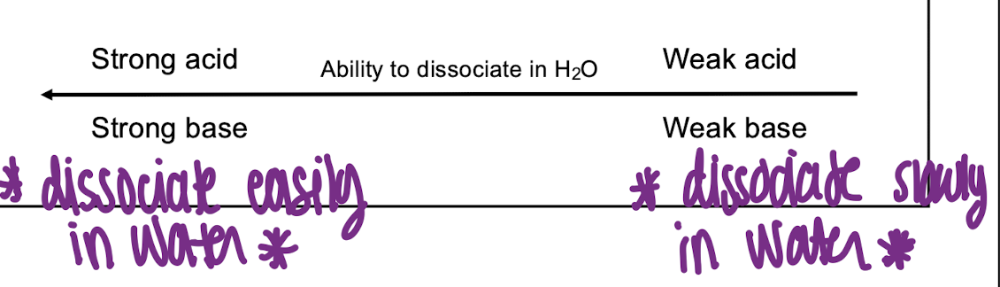 F |
front 3 T/F: Strong acid readily dissociates in water | back 3  T |
front 4 T/F: [H+] does not vary a lot but [Na+] does | back 4  F |
front 5 T/F: Urine pH can be as low as 4.5 | back 5 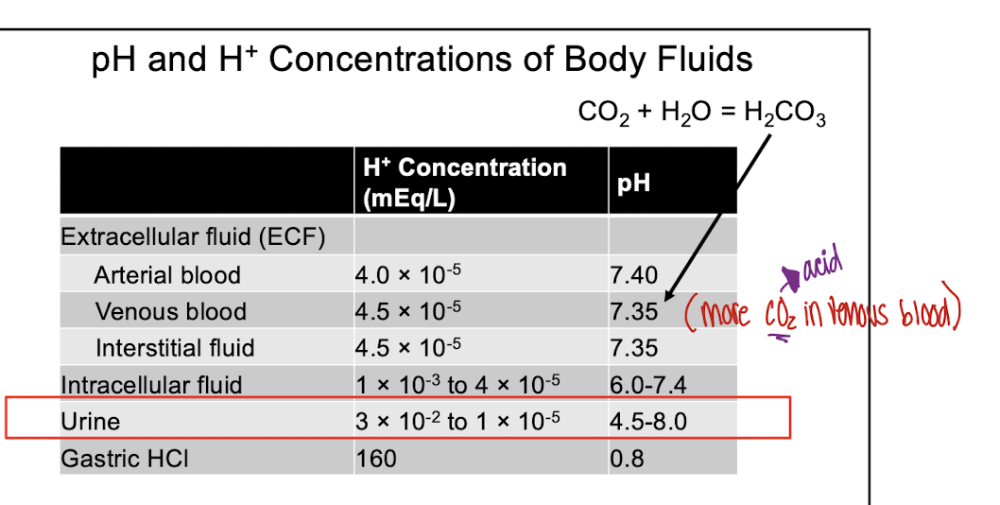 T |
front 6 T/F: Buffering urine is essential for our physiological functions | back 6 F |
front 7 T/F: Net uptake of acid from dietary source is 40 mmole/day | back 7 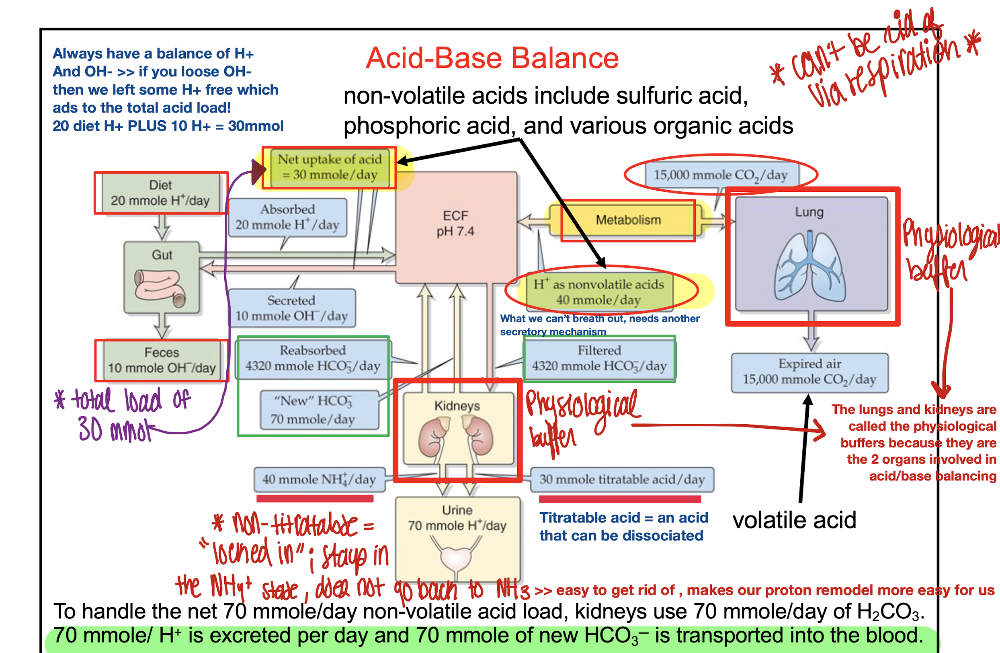 F Net dietary intake is 20mmol/day |
front 8 T/F: Non-volatile acids are removed from our body through expiration | back 8  F They can't be breathed out and therefore need another secretory mechanism for removal |
front 9 T/F: Alteration of [H+] influences rate of biochemical reactions, without affecting the distribution of other ions | back 9  F There WILL BE an alteration of the distribution of ions |
front 10 T/F: Alteration of [H+] is momentarily corrected by respiratory system | back 10 F |
front 11 What are the 3 primary systems to regulate h+ in body fluids? | back 11 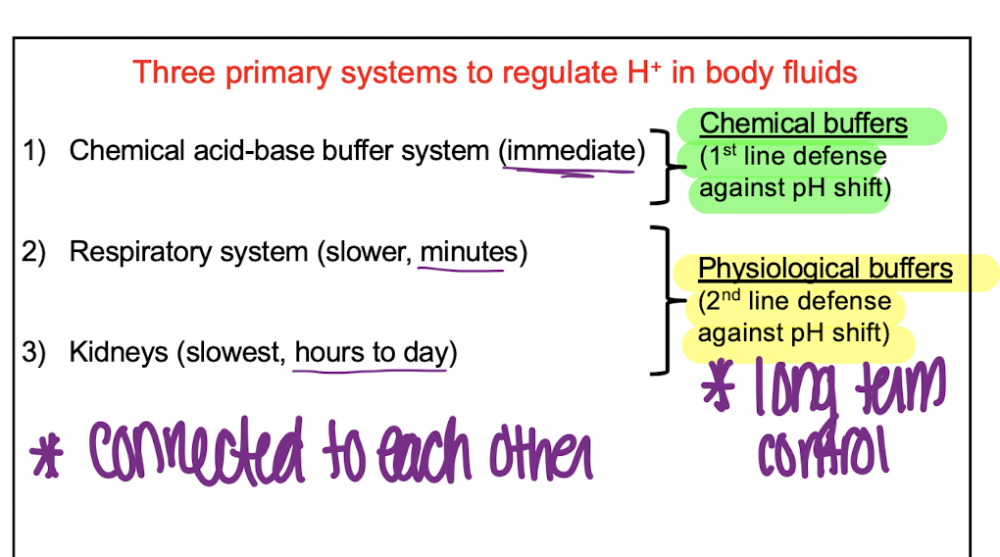 |
front 12 What are the 4 major Chemical Acid-Base buffer systems (immediate responses)? (BPAP) | back 12
|
front 13 T/F: Bicarbonate buffer system is the most important system for buffering extracellular fluids (plasma) | back 13 T Bicarbonate buffer system contains
|
front 14 T/F: Phosphate and ammonia buffer systems are important in renal tubules | back 14 T
|
front 15 T/F: Proteins are involved in majority of chemical buffering in body fluids | back 15 T Protein buffer system is the MOST PLENTIFUL BUFFERS In the body and important for intracellular fluid buffering |
front 16 What happens to CO2 levels when buffering bases? | back 16 CO2 levels decreased - leading to decreased respiration |
front 17 Buffer Power is dependent on what 2 factors? | back 17
|
front 18 T/F: When buffering strong acid, CO2 level increases | back 18 T Leads to increased expiration |
front 19 Which of the following statements is not true about acid-base balance? A.Net uptake of H+ through GI system is 30 mmole/day B.Non-volatile acids are removed from our body through kidneys C.Alteration of [H+] is momentarily corrected by respiratory system D.Buffering urine is not essential for our physiological functions | back 19 C.Alteration of [H+] is momentarily corrected by respiratory system |
front 20 Q. All of the following statements are true about bicarbonate buffer systems EXCEPT: A.CO2 levels increase when buffering acids, leading to decreased expiration B.CO2 levels decrease when buffering bases, leading to decreased respiration C.Generated HCO3- remains in the system, but HCO3- salts are excreted by the kidneys D.Carbonic anhydrase is not present in the blood plasma so the formation of carbonic acid is very slow in plasma | back 20  A.CO2 levels increase when buffering acids, leading to decreased expiration |
front 21 A DECREASE in ventilation rate = ___________ in pCO2 | back 21 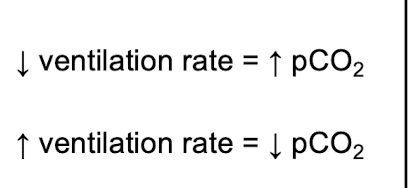 INCREASE |
front 22 AN INCRESE in ventilation rate = ______________________ in pCO2 | back 22 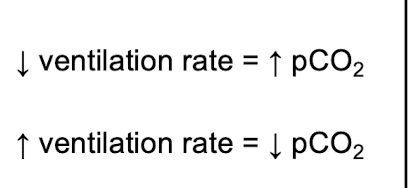 DECREASE |
front 23 Doubling the ventilation rate increases pH by how much? | back 23 0.23 |
front 24 Q. Which of the following is/are paired with the correct rate? A.Chemical acid base buffer systems: slower, minutes B.Kidneys: slowest, hours to days C.Respiratory system: immediate D.A and B | back 24  B.Kidneys: slowest, hours to days |
front 25 T/F: We vary urine pH within 4.5 – 8.0 so that we can maintain blood pH within the narrow range of 7.35 – 7.45. | back 25 T |
front 26 Q. Which is the most important system for buffering extracellular fluids? A.Phosphate buffer system B.Bicarbonate buffer system C.Ammonia buffer system D.Protein buffer system | back 26 B.Bicarbonate buffer system |
front 27 Q. Ammonium ion (NH4+) is best described as what kind of acid? A.Volatile acid B.Titratable acid C.Non-titratable acid D.None of the above | back 27 C.Non-titratable acid |
front 28 Q. Which of the following is NOT true about ammonia buffer system? a.NH4+ is a non-titratable acid b.Ammonia buffer system is important in renal tubule c.Ammonia buffer system generates new HCO3─ d.Conversion of NH4+ to NH3 can easily take place at urine pH | back 28 d.Conversion of NH4+ to NH3 can easily take place at urine pH False, because NH4+ is non-titratable meaning it is irreversible |
front 29 Q. Which major system is the most plentiful in the body and important for intracellular fluid buffering? A.Bicarbonate buffer system B.Phosphate buffer system C.Protein buffer system D.Ammonia buffer system | back 29 C.Protein buffer system Intracellular proteins account for 60-70% of chemical buffering in body fluids. Highest concentrations in intracellular fluid. Not very good buffers in urine. |
front 30 Q. Which of the following statements is incorrect regarding the phosphate buffer system? A.This buffer system uses H2PO4- and HCO3- B.Phosphate buffer system plays an important role in buffering renal tubule and intracellular fluids C.Phosphate is important in renal tubules because it is greatly concentrated there D.Phosphate buffer system is not the most important buffer system in blood | back 30 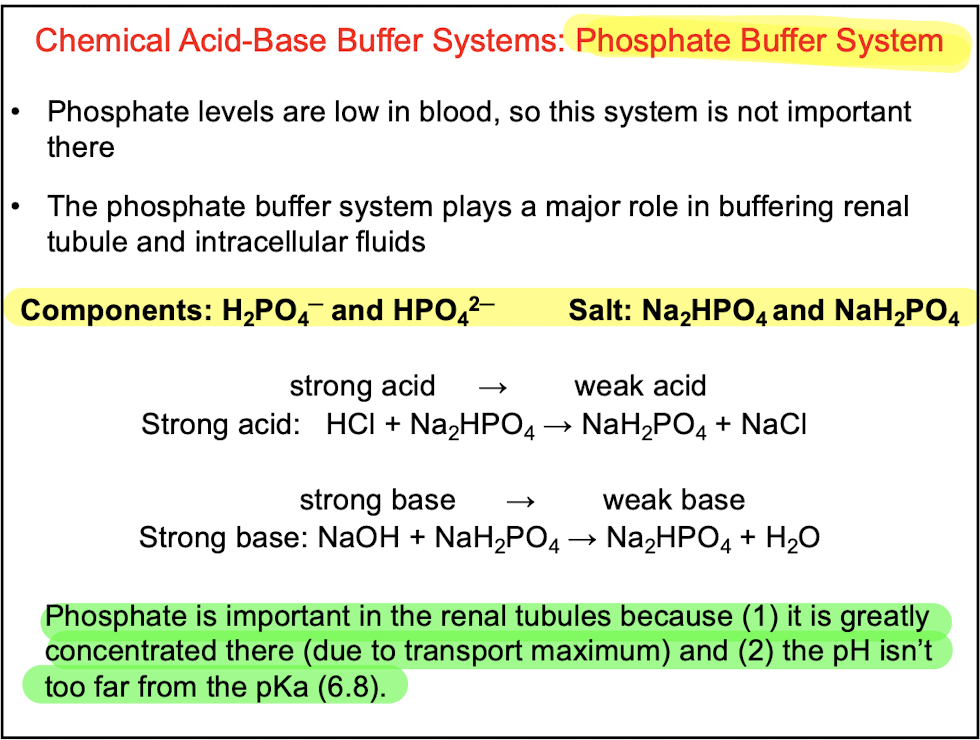 A.This buffer system uses H2PO4- and HCO3- |
front 31 Q. What is the important buffering system in the renal tubules that is subject to physiological control? A.Bicarbonate buffer system B.Phosphate buffer system C.Protein buffer system D.Ammonia buffer system | back 31 D.Ammonia buffer system Components: NH 3 and NH 4 + , a mechanism for making new HCO3 - |
front 32 Q. CO2 levels increase when buffering acids leading to ______________ expiration. A.increased B.decreased C.no change D.none of the above | back 32 A.increased |
front 33 Q. Which of the three primary systems that regulates H+ in body fluids is the slowest? A.Chemical acid-base buffer system B.Respiratory system C.Kidneys D.B and C | back 33 C.Kidneys Kidneys control acid-base balance by excreting either basic or acidic urine |
front 34 Q. All of the following are mechanisms by which extracellular H+ is regulated EXCEPT: A.Production of new H2PO4- B.Secretion of H+ C.Reabsorption of filtered HCO3- D.Production of new HCO3- | back 34  A.Production of new H2PO4- |
front 35
| back 35
|
front 36 To remove the 70 mEq of H+ generated by the body each day, we would need to excrete more than _________ of urine a day | back 36 2300 L The kidneys can excrete H+, as much as 500 mEq/day, using buffers |
front 37 Filtered load of HCO3 - is _______ moles/day | back 37 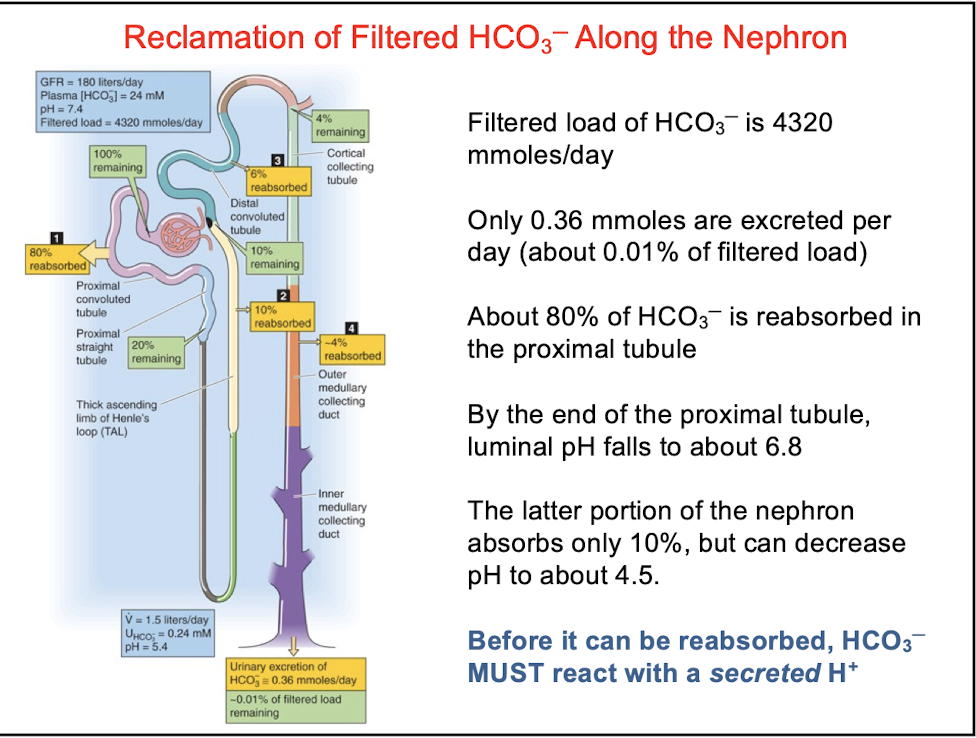 4320 |
front 38 Only __________ moles are excreted per day along the nephron | back 38  0.36 |
front 39 About _____ of HCO3 - is reabsorbed in the proximal tubule | back 39  80% reabsorption of HCO3 - requires secretion of H+ = one H+ secreted into the lumen > one HCO3 - enters the blood |
front 40 The 2 major mechanisms for HCO3 - regeneration along the nephron? | back 40
|
front 41 _____________ is the primary pH buffer in normal urine | back 41 Phosphate |
front 42 INCREASING phosphate in urine will _______________ urine acidity | back 42 increase |
front 43 Phosphate is reabsorbed by what kind of transporter? | back 43 Na+/phosphate transporter |
front 44 Q. What is the function of Type B intercalated cells of the collecting ducts? Select All that apply A.HCO3- reabsorption B.H+ reabsorption C.HCO3- secretion D.H+ secretion | back 44  B.H+ reabsorption C.HCO3- secretion |
front 45 Q. A decreased ventilation rate is a compensatory mechanism for: A.Decrease in pCO2 B.Increase in HCO3- C.Increase in pCO2 D.Decrease in HCO3- | back 45 B. Increase in HCO3- |
front 46 Q. Which of the following is NOT a major three primary buffer systems to regulate H+? A.Chemical acid base buffer system B.Respiratory system C.Kidneys D.Phosphate buffer system | back 46 D.Phosphate buffer system |
front 47 Q. How will the presence of a strong acid affect ventilation when using the HCO3- system? A.Hyperventilation B.Hypoventilation C.Normal ventilation D.None of the above | back 47 A.Hyperventilation |
front 48 Q. CO2 levels ________ when buffering strong bases leading to a/an __________ in respiration. A.increase, decrease B.decrease, decrease C.increase, increase D.decrease, increase | back 48 B.decrease, decrease |
front 49 T/F: Increases in CO2 decrease alveolar ventilation | back 49 F |
front 50 T/F: Buffering strong acids increases alveolar ventilation | back 50 T |
front 51 T/F: Decreases in CO2 decrease alveolar ventilation | back 51 T |
front 52 T/F: NH4+ is a titratable acid | back 52 F |
front 53 T/F: Ammonium buffer system reduces HCO3- in the body | back 53 F |
front 54 T/F: Protein buffer system makes new HCO3- | back 54 F Ammonia buffer system makes the new HCO3 - |
front 55 T/F: Phosphate buffer system is an example of physiological buffer | back 55 F |
front 56 T/F: For every HCO3─ to be reabsorbed, it must react with a secreted H+ | back 56 T |
front 57 T/F: Type A intercalated cells secrete HCO3─ | back 57  F |
front 58 T/F: Type B intercalated cells reabsorb H+ | back 58  T |
front 59 T/F: Type B intercalated cells have HCO3─/Cl ─ transporter on the apical membrane H+ | back 59  T |
front 60 T/F: Type A intercalated cells have H+ pump on the apical membrane H+: | back 60  T |
front 61 T/F: Phosphate buffer removes one H+ while reabsorbing one HCO3─ | back 61 T |
front 62 T/F: Glutamine breakdown in the renal tubules removes one H+ and produces one HCO3 - | back 62 F For every glutamine molecule metabolized, two NH4+ are secreted into the lumen and two HCO3 - are reabsorbed into the blood. |
front 63 In Acidosis: H+ secretion is _________________, HCO3 - reabsorption is ____________, titratable acid and NH4 + are ______________, no new ______ is made. | back 63 H+ secretion is decreased, HCO3 - reabsorption is incomplete, titratable acid and NH4 + are not excreted (no excess H+), no new HCO3 - is made |
front 64 In alkalosis: H+ secretion allows ________ of HCO3 - , ________ of NH4 + and titratable acid, adding new __________ to the body | back 64 H+ secretion allows reabsorption of HCO3 - , excretion of NH4 + and titratable acid, adding new HCO3 - to the body |
front 65 What are the respiratory acid-base disturbances related to pCO2 levels? | back 65  |
front 66 What are the metabolic acid-base disturbances related to intracellular HCO3 - levels? | back 66  |
front 67 Diagnosis of disturbances in Acid-Base Balance: relationship between pCO2 and HCO3 - (not a question, just a lecture slide) | back 67 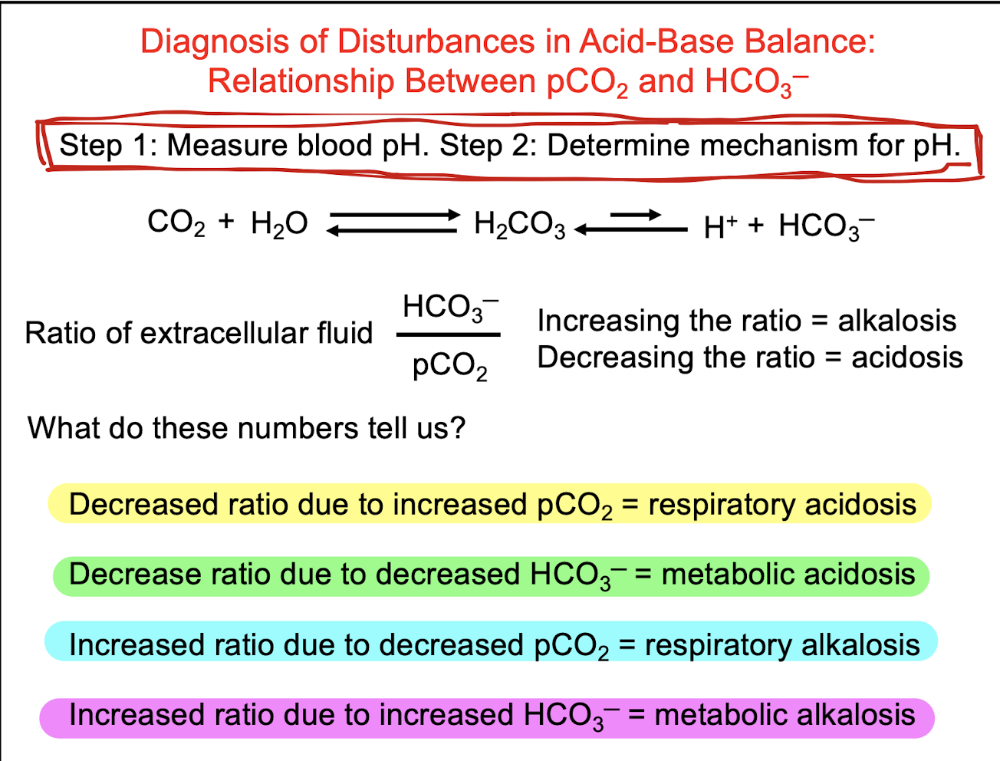 |
front 68 T/F: HCO3─ increases in metabolic acidosis | back 68 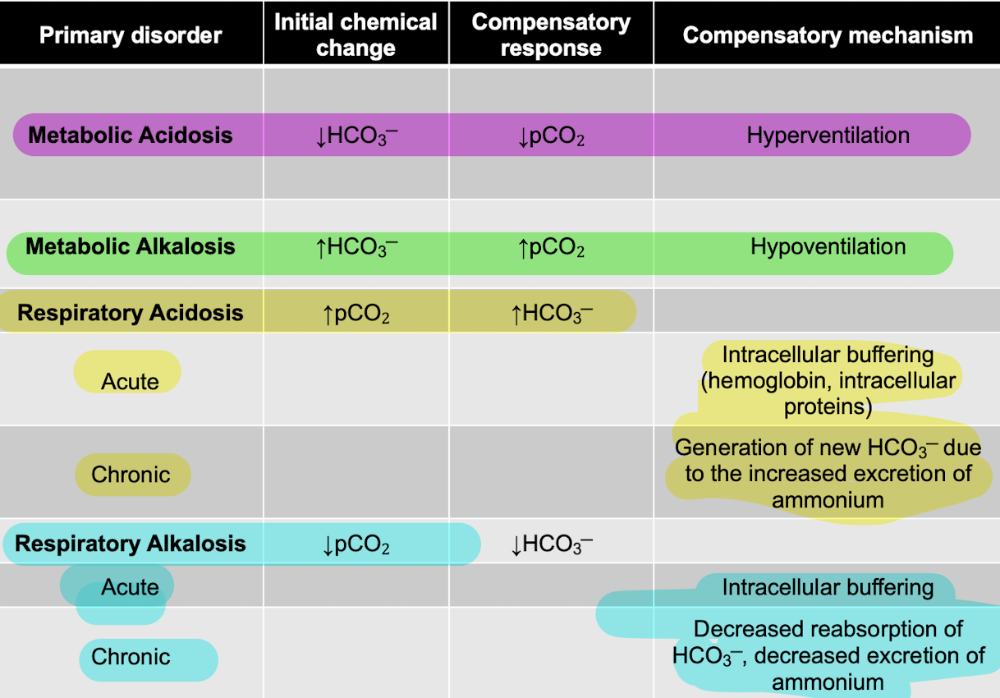 F |
front 69 The body must maintain electroneutrality, meaning what? | back 69 the Total concentration of cations in the serum must equal the total concentration of anions [Na+] + [UCs] = ([Cl-] + ]HCO3 -]) + [UAs]
|
front 70 What is the Anion gap ? | back 70 The difference in the serum (plasma) concentrations of the "measured" cations and anions
|
front 71 Causes of Elevation Anion Gap:
| back 71  |
front 72 What is the Anion gap equation? | back 72 Anion gap = [Na+] – ([Cl-] + [HCO3 -]) |
front 73 T/F: The end result in type A intercalated cell action is one H+ ion secreted into the lumen and bicarbonate reabsorbed into the blood stream. | back 73 True |
front 74 Q. Type B intercalated cells in the collecting duct secrete one _______ into the lumen and reabsorb one______ into the bloodstream a.H+, HCO3─ b.HCO3─, K+ c.HCO3─, H+ d.NH3, H+ | back 74 c.HCO3─, H+ |
front 75 Q. Choose the correct statement as it relates to pH and ventilation. A.As blood pH decreases, alveolar ventilation increases B.As blood pH increases, alveolar ventilation increases C.As blood pH decreases, alveolar ventilation decreases D.As blood pH decreases, alveolar ventilation stops | back 75 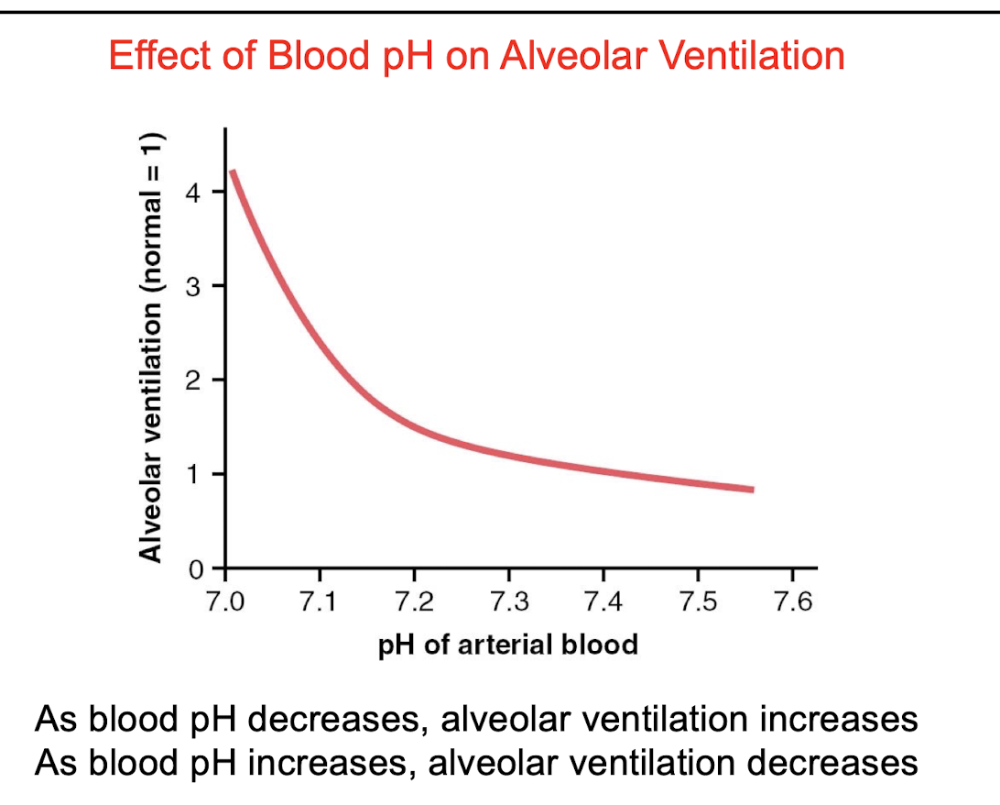 A. As blood pH decreases, alveolar ventilation increases |
front 76 Q. Which buffering mechanism(s) removes non-volatile acids and generates HCO3-? A.Phosphate B.Bicarbonate C.Ammonia D.Protein | back 76 A.Phosphate A. Ammonia |
front 77 Q. Which of the following are the PRIMARY mechanisms by which the kidneys can regulate blood pH? (Select all that apply) A.Secretion of H+ B.Reabsorption of H+ C.Production of new HCO3- D.Reabsorption of filtered HCO3- | back 77  A.Secretion of H+ C.Production of new HCO3- D.Reabsorption of filtered HCO3- |
front 78 Q. All of the following are the causes of elevated anion gap except: A.Uremia B.Ketoacidosis C.Lactic acidosis D.Hyperchloremia | back 78  D.Hyperchloremia |
front 79 Q. What is metabolic acidosis? a.increase in CO2 level in the body b.decrease in CO2 level in the body c.increase in HCO3─ level in the body d.decrease in HCO3─ level in the body | back 79  d.decrease in HCO3─ level in the body |
front 80 Q. Metabolic acidosis results from a(n) __________ in blood pH due to decreased bicarbonate levels. To compensate for this change in pH, carbon dioxide levels will __________ through __________. a.increase, decrease, hyperventilation b.decrease, decrease, hyperventilation c.decrease, decrease, hypoventilation d.decrease, increase, hypoventilation | back 80 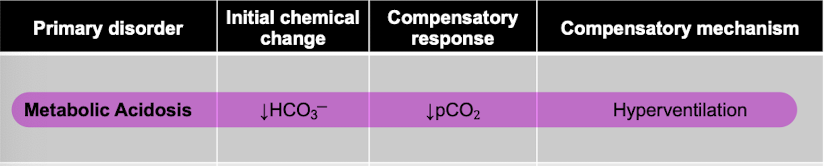 b.decrease, decrease, hyperventilation |
front 81 Q. Which of the following mechanisms can cause alkalosis? Select all that apply. A.excess addition of H+ to the body fluids. B.excess removal of H+ from the body fluids C.excess addition of HCO3- to the body fluids. D.excess removal of HCO3- to the body fluids. | back 81 B.excess removal of H+ from the body fluids C.excess addition of HCO3- to the body fluids. |
front 82 Q. For every glutamine molecule catabolized, ___________ are secreted into the lumen and__________ are reabsorbed into the blood. A.1 NH4+, 1 HCO3─ B.2 NH4+, 2 HCO3─ C.2 H+, 2 HCO3─ D.1 H+, 1 HCO3─ | back 82 B.2 NH4+, 2 HCO3─ |
front 83 Q. Which buffer system(s) removes H+ and generates HCO3─? Select ALL that apply a.Bicarbonate buffer system b.Ammonia buffer system c.Phosphate buffer system d.Protein buffer system | back 83 b.Ammonia buffer system c.Phosphate buffer system |
front 84 Q. What is the correct equation used to describe the anion gap? A.[Na+] + ([Cl-] + [HCO3-]) B.[Na+] + ([Cl-] - [HCO3-]) C.[Na+] - ([Cl-] + [HCO3-]) D.[Na+] - [Cl-] - [HCO3-] | back 84 C.[Na+] - ([Cl-] + [HCO3-]) D.[Na+] - [Cl-] - [HCO3-] |
front 85 Q. Which of the following is consistent with metabolic acidosis? a.Decreased HCO3 - b.Increased unmeasured anions c.Increased anion gap d.All of the above | back 85 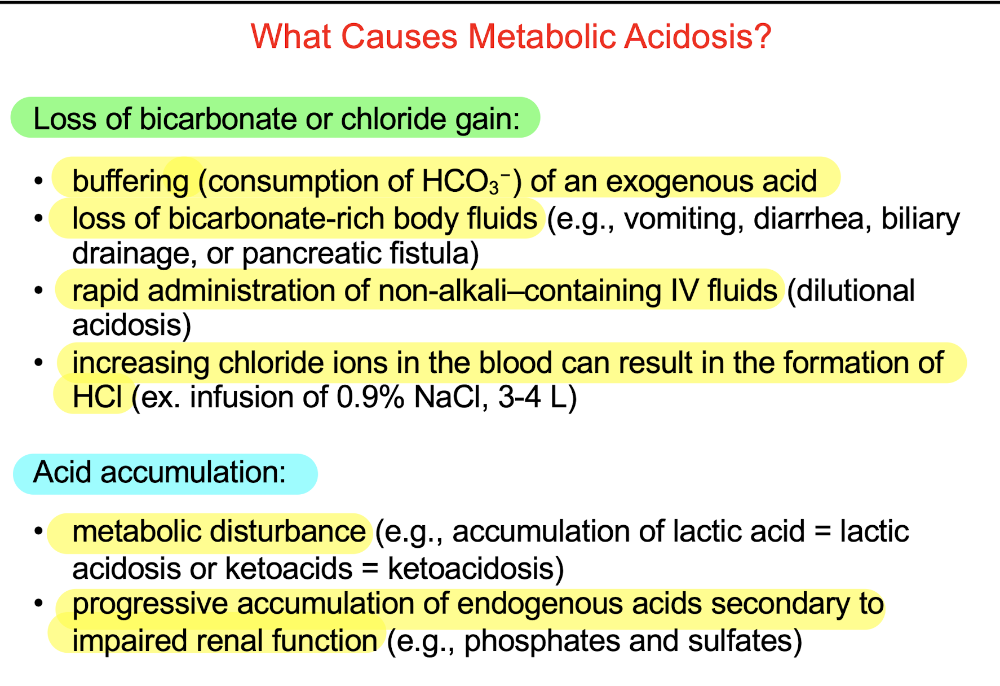 d.All of the above |
front 86 Q. With bicarbonate buffer system, what happens to levels of CO2 when buffering a strong acid to a weak acid? What happens to expiration? A.Increase, Increase B.Decreases, Increase C.Decrease, Decrease D.Increase, Decrease | back 86 A.Increase, Increase |
front 87 Q. What happens to pCO2 levels when a patient hypoventilates? A.Remains the same B.Increases C.Decreases D.Doubles | back 87 B. Increases |
front 88 Q. What happens in the bicarbonate buffer system when excess amounts of NaOH are added to the system? A.NaHCO3 formation and increased CO2 B.NaHCO3 formation and decreased CO2 C.Increased CO2 and H2O D.No change in CO2 or NaHCO3 formation | back 88 B.NaHCO3 formation and decreased CO2 |
front 89 Q. There are three primary systems that regulate H+ in body fluids. Rank them according to the time it takes (slowest to fastest). A.Kidneys - Respiratory - Chemical acid-base buffer systems B.Respiratory - Kidneys - Chemical acid-base buffer systems C.Respiratory - Chemical acid-base buffer systems - Kidneys D.Chemical acid-base buffer systems - Respiratory - Kidneys | back 89 A.Kidneys - Respiratory - Chemical acid-base buffer systems |
front 90 Q. When you have a serum anion gap value that is normal during metabolic acidosis, what is the likely status of chloride in serum? A.Hyperchloremic B.Hypochloremic C.Normal values D.There is no chloride present in blood | back 90 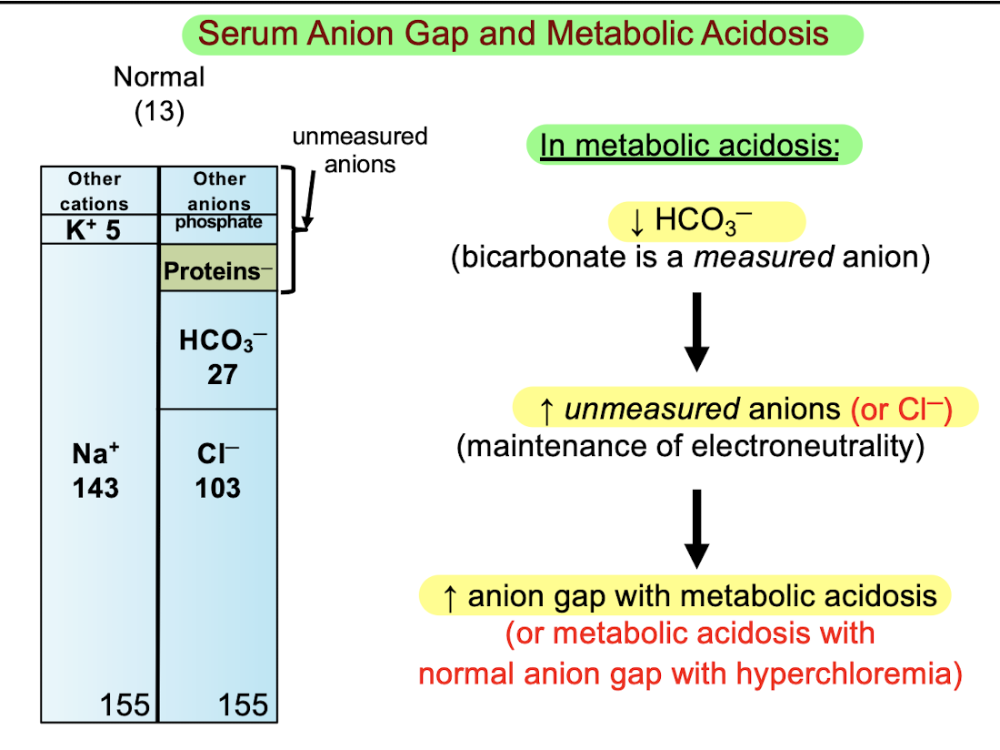 A.Hyperchloremic |
front 91 Q. While the ______ buffering system accounts for the majority of the buffering in body fluids, the _______ buffering system is better at buffering plasma pH. A.phosphate ; bicarbonate B.ammonia; bicarbonate C.protein; bicarbonate D.bicarbonate; phosphate | back 91 C.protein; bicarbonate |
front 92 Q. For the diagnosis of acid/base imbalance which of the following statements is FALSE when dealing with the ratio of extracellular fluid: HCO3-/pCO2 ? A.Decreased ratio due to increased pCO2 = respiratory acidosis B.Decrease ratio due to decreased HCO3- = metabolic alkalosis C.Increased ratio due to decreased pCO2 = respiratory alkalosis D.Increased ratio due to increased HCO3- = metabolic alkalosis | back 92  B.Decrease ratio due to decreased HCO3- = metabolic alkalosis |
front 93 Q. All of the following are common with titration acidosis except: A.HCO3- level decreases B.Anion gap increases C.HCO3- consumed by a noncarbonic acid D.Cl- level decreases | back 93 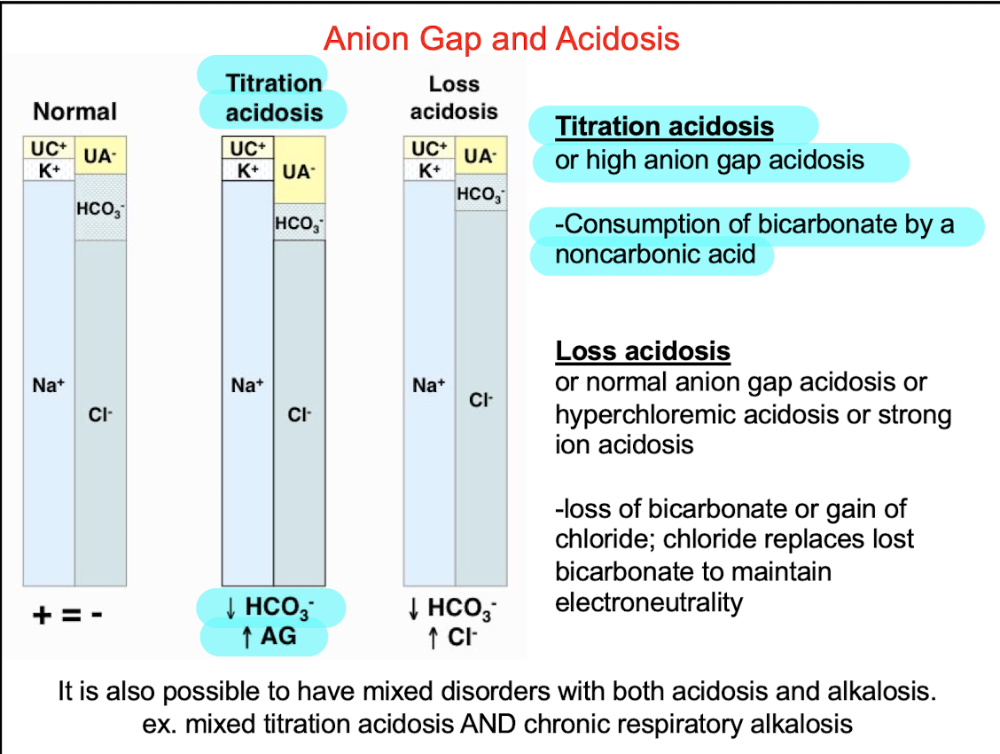 D. Cl- level decreases |
front 94 Q. What is the compensatory mechanism of metabolic acidosis? A.Hyperventilation B.Hypoventilation C.Intracellular buffering D.Generation of new HCO3- because of ammonia excretion | back 94  A.Hyperventilation |