How many grams of oxygen (O2) are needed to completely react with 24.0 g of propane (C3H8) according to the equation below?
a. 0.545 g
b. 2.73 g
c. 87.2 g
d. 28.8 g
Catalysts lower the activation energy of a chemical reaction.
True
False
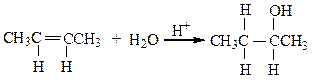
The reaction below is an example of a ___ reaction.
a. hydrogenation
b. hydrolysis
c. dehydration
d. hydration
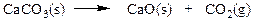
Classify the reaction below as involving synthesis, decomposition, single replacement, or double replacement.
a. single replacement
b. double replacement
c. decomposition
d. synthesis

Which set of coefficients properly balance the equation below, going from reactants to products?
a. 1,3,2,3
b. 1,7,4,3
c. 2,7,4,6
d. 2,3,2,3
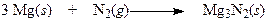
Regarding the following reaction,
a. This reaction does not involve oxidation or reduction.
b. Magnesium is oxidized and nitrogen is reduced.
c. Both magnesium and nitrogen are reduced.
d. Magnesium is reduced and nitrogen is oxidized.
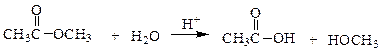
In the following reaction, how many grams of water are needed to completely hydrolyze 30.8 g of the ester?
a. 7.48 g
b. 0.416 g
c. 30.8 g
d. 11.3 g
What was the earliest reported method of fighting infection in the operating room?
a. The patient was treated with Betadine before surgery.
b. Carbolic acid was used on the surgeon’s tools.
c. Listerine was the antiseptic of choice.
d. The surgeons washed with soap and chlorine water.
Ethene, C2H4, burns in air according to the equation below. What is the theoretical yield (in grams) of CO2 when 3.24 g of C2H4 are reacted with 4.83 g of O2?
a. 3.24 g
b. 9.72 g
c. 0.151 g
d. 4.43 g
An oxidation reaction can be defined as when an atom loses an oxygen.
True
False
2-methylcyclopentene reacts with hydrogen in the presence of platinum catalyst to form methylcyclopentane. In this reaction:
a. 2-methylcyclopentene is reduced
b. Pt is oxidized
c. methylcyclopentane is reduced
d. cyclohexane is oxidized
The function of an enzyme in a reaction is to
a. cause a reaction to be reversible at the same reaction rate.
b. increase the ∆G to increase the rate of reaction.
c. increase the rate of reaction by reducing the activation energy.
d. inhibit the reaction.
The subscripts of a chemical formula can be changed to balance a chemical equation.
True
False
Benzoyl peroxide can be used as a treatment for acne. Benzoyl peroxide
a. has a reduction action that tends to neutralize the effects of the acne bacteria.
b. is injected so that it will have a general immune action against bacteria causing acne.
c. is an oxidizing agent used on the skin to kill the bacteria causing acne.
d. is not used for acne; it is an antiseptic used to sterilize a surgeon’s hands.
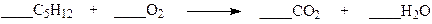
Which set of coefficients properly balance the equation below?
a. 1,1,5,6
b. 1,5,5,6
c. 1,5,5,1
d. 1,8,5,6
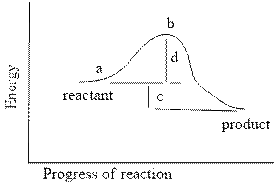
In the reaction energy diagram below, the activation energy is represented by:
a. c
b. a
c. d
d. c
What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine?
a. 5.2 g KCl
b. 4.9 g KCl
c. 9.9 g KCl
d. 10.1 g KCl
Which reaction is NOT an oxidation-reduction reaction?
a. b. c. d. All of these are oxidation-reduction reactions.
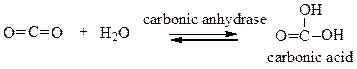
Choose the comment that is appropriate for the reaction:
a. Carbonic anhydrase catalyzes the conversion of carbon dioxide into carbonic acid.
b. The reaction runs in forward direction only.
c. This reaction runs in backward direction only.
d. This is a reaction that only occurs in the laboratory.
Methanol burns in air according to the equation below. How many grams of methanol can be burned by 10 grams of oxygen?
a. 2 g
b. 7 g
c. 10 g
d. 20 g
When the pressure is increased above 760 torr, the boiling point of water
a. decreases to temperatures below 100 C.
b. remains the same at 100C.
c. increases to temperatures above.
d. drops to temperatures below 0C.
Ethyl alcohol (CH3CH2OH) is completely soluble in water. It is made of both polar and non-polar groups. Ethyl alcohol is best classified as a(n) ___ substance.
a. hydrophilic
b. hydrophobic
c. lipophilic
d. amphipathic
What is the volume of a mole of an ideal gas under standard conditions (STP)?
a. 0.0821 liter
b. 760 liters
c. 22.4 liters
d. 1.0 liter
The gas constant is used in some calculations. The value of the gas constant is
a. to make certain that all gases are ideal gases.
b. 0.0821 L atm/mol K.
c. 25°C and 1 atm.
d. 0°C and 1 atm at 1-L volume.
Identify the compound that is insoluble in water.
a. Ba3(PO4)2
b. Ba(NO3)2
c. K3PO4
d. AgNO3
In a mountain location where the atmospheric pressure is less than 1.00 atm, water will boil at ___.
a. a temperature less than 100ºC
b. 100ºC
c. a temperature greater than 100ºC
d. None of these choices.
How many milliliters of 6.0 M HCl must be diluted to make 500.0 mL of a 0.10 M solution?
a. 1.2 mL
b. 8.3 mL
c. 30. mL
d. 300 mL
The atmospheric pressure is greater at the top of the mountain than in the valley below.
True
False
A cylinder of gas originally at 28.0°C and 1.00 atm of pressure is heated to 112.0°C. What is the new pressure?
a. 4.00 atm
b. 0.250
. 0.782 atm
d. 1.28 atm
Which of these contains the largest particles?
a. solution
b. colloid
c. suspension
d. solvent
Red blood cells will shrink in a hypotonic solution.
True
False
A patient’s blood test indicates that 1.00 mL of serum contains 0.00140 g of fibrinogen (a protein that aids in coagulation of blood). What is the concentration of the fibrinogen in mg/dL?
a. 280 mg/dL
b. 14 mg/dL
c. 0.014 mg/dL
d. 140. mg/dL
Blood serum has a calcium ion (Ca2+) concentration of 3.9
x 10-4
M. What is the concentration in mEq/L?
a. 0.78 mEq/L
b. 0.39 mEq/L
c. 2.3 mEq/L
d. 6.8 mEq/L
If red blood cells were to be stored in a hypertonic solution in the blood bank, then
a. there would be no problems as this matches the red blood cell’s composition
.b. the cells would tend to explode due to the movement of water into the cells.
c. the water would flow out of the cells resulting in shrinkage and wrinkling.
. there would be too much water present to store red blood cells effectively.
In a homogeneous mixture
a. the substances are not evenly distributed.
b. the substances are always liquid.
c. the substances are always solids.
d. the substances are uniformly distributed.
15.0 g of KCl is dissolved into a solution with a total volume of 250.0 mL. What is the molarity of the solution?
a. 0.200 M
b. 3.75 M
c. 0.804 M
d. 1.24 M
How many grams of AgNO3 are required to make 500.0 mL of a 0.10 M solution?
a. 8.5 g
b. 17 g
c. 85 g
d. 0.10 g
A prodrug is
a. used because it is less expensive than the regular medication.
b. a more professional type of medication than is normally used.
c. the metabolic result of normal bodily processes on commonly available materials.
d. a precursor of a drug that is converted by metabolic processes to that drug.
A sample of neon gas fills a 6.85-L container. What is the new temperature (in Kelvin) if the gas is transferred into a 9.64-L container and pressure adjusted to 604 torr?
a. 305 K
b. 483 K
c. 244 K
d. 656 K
A gas occupies a volume of 8.60 L at 45C and 764 torr. What pressure would it occupy if the volume is adjusted to 13.3 L at 20C?
a. 536 torr
b. 455 torr
c. 109 torr
d. 188 torr
Pentose, C5H10O5, reacts with oxygen to produce carbon dioxide and water. What is the coefficient on O2 when the equation below is properly balanced?
a. 3
b. 5
c. 7
d. 15
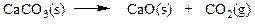
Which set of coefficients properly balance the equation below, going from reactants to products?
a. 1,2,1,4
b. 2,1,1,2
c. 2,2,3,1
d. 1,2,2,1
An oxidizing agent is the reactant in an oxidation - reduction reaction that causes reduction of another reactant by providing electrons for the other reactant to accept.
True
False
Balance the equation below and determine the number of moles of oxygen required to completely burn 5.00 moles of propane.
a. 5.00
b. 10.0
c. 15.0
d. 25.0
When ∆G for a reaction is +286 kcal, the reaction is spontaneous.
True
False
While this isn’t a real reaction, we can say that carbonic acid, H2CO3, can be produced directly from the elements. Choose the statement that is correct when the given masses are supplied to react.
a. Hydrogen is the limiting reactant.
b. Carbon is the limiting reactant.
c. Oxygen is the limiting reactant.
d. There is no limiting reactant.
What will you observe if you mix a solution of KOH with a solution of MgI2
in the reaction below?
. bubbles of KI will form
b. a solid of Mg(OH)2 will settle in reaction vessel
c. a solid of KOH will form
d. a clear solution will form
Two methanol molecules can react in the presence of sulfuric acid to produce dimethyl ether. Calculate the percent yield for this reaction that was run in a laboratory starting with 50 grams methanol and producing 26 grams dimethyl ether.
a. 52%
b. 57%
c. 72%
d. 200%
What mass of potassium chloride, a salt substitute often used by heart patients, can be produced directly from 5.2 g potassium and 7.9 g chlorine?
a. 5.2 g KCl
b. 4.9 g KCl
c. 9.9 g KCl
d. 10.1 g KCl
Whipped cream is an example of a ___.
a. solution
b. suspension
c. colloid
d. solvent