Which of the following is true of metabolism in its entirety in all organisms?
A) Metabolism depends on a constant supply of energy from food.
B) Metabolism uses all of an organism's resources.
C) Metabolism consists of all the energy transformation reactions in an organism.
D) Metabolism manages the increase of entropy in an organism.
C) Metabolism consists of all the energy transformation reactions in an organism.
Which of the following is an example of potential rather than kinetic energy?
A) water rushing over Niagara Falls
B) light flashes emitted by a firefly
C) a molecule of glucose
D) a crawling beetle foraging for food
C) a molecule of glucose
Most cells cannot harness heat to perform work because _____.
A) heat is not a form of energy
B) temperature is usually uniform throughout a cell
C) heat can never be used to do work
D) heat must remain constant during work
B) temperature is usually uniform throughout a cell
Which of the following involves a decrease in entropy?
A) condensation reactions
B) reactions that separate monomers
C) depolymerization reactions
D) hydrolysis reactions
A) condensation reactions
Which term most precisely describes the cellular process of breaking down large molecules into smaller ones?
A) catabolism (catabolic pathways)
B) metabolism
C) anabolism (anabolic pathways)
D) dehydration
A) catabolism (catabolic pathways)
Anabolic pathways _____.
A) are usually highly spontaneous chemical reactions
B) consume energy to build up polymers from monomers
C) release energy as they degrade polymers to monomers
D) consume energy to decrease the entropy of the organism and its environment
B) consume energy to build up polymers from monomers
Which of the following is a statement of the first law of thermodynamics?
A) Energy cannot be created or destroyed.
B) The entropy of the universe is decreasing.
C) The entropy of the universe is constant.
D) Energy cannot be transferred or transformed.
A) Energy cannot be created or destroyed.
For living organisms, which of the following is an important consequence of the first law of thermodynamics?
A) The energy content of an organism is constant.
B) The organism ultimately must obtain all of the necessary energy for life from its environment.
C) The entropy of an organism decreases with time as the organism grows in complexity.
D) Organisms grow by converting energy into organic matter.
B) The organism ultimately must obtain all of the necessary energy for life from its environment.
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
A) Living organisms do not obey the second law of thermodynamics, which states that entropy must increase with time.
B) Life obeys the second law of thermodynamics because the decrease in entropy as the organism grows is exactly balanced by an increase in the entropy of the universe.
C) As a consequence of growing, organisms cause a greater increase in entropy in their environment than the decrease in entropy associated with their growth.
D) Living organisms are able to transform energy into entropy.
C) As a consequence of growing, organisms cause a greater increase in entropy in their environment than the decrease in entropy associated with their growth.
Which of the following statements is a logical consequence of the second law of thermodynamics?
A) If the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe.
B) If there is an increase in the energy of a system, there must be a corresponding decrease in the energy of the rest of the universe.
C) Every chemical reaction must increase the total entropy of the universe.
D) Energy can be transferred or transformed, but it cannot be created or destroyed.
C) Every chemical reaction must increase the total entropy of the universe.
Which of the following statements is representative of the second law of thermodynamics?
A) Conversion of energy from one form to another is always accompanied by some gain of free energy.
B) Without an input of energy, organisms would tend toward decreasing entropy.
C) Cells require a constant input of energy to maintain their high level of organization.
D) Every energy transformation by a cell decreases the entropy of the universe.
C) Cells require a constant input of energy to maintain their high level of organization.
Which of the following types of reactions would decrease the entropy within a cell?
A) anabolic reactions
B) hydrolysis
C) digestion
D) catabolic reactions
A) anabolic reactions
Biological evolution of life on Earth, from simple prokaryote-like cells to large, multicellular eukaryotic organisms, _____.
A) has occurred in accordance with the laws of thermodynamics
B) has caused an increase in the entropy of the planet
C) has been made possible by expending Earth's energy resources
D) has occurred in accordance with the laws of thermodynamics, by expending Earth's energy resources and causing an increase in the entropy of the planet
A) has occurred in accordance with the laws of thermodynamics
The mathematical expression for the change in free energy of a system is ΔG =ΔH - TΔS. Which of the following is (are) correct?
A) ΔS is the change in enthalpy, a measure of randomness.
B) ΔH is the change in entropy, the energy available to do work.
C) ΔG is the change in free energy.
D) T is the temperature in degrees Celsius.
C) ΔG is the change in free energy.
A system at chemical equilibrium _____.
A) consumes energy at a steady rate
B) releases energy at a steady rate
C) has zero kinetic energy
D) can do no work
D) can do no work
Which of the following is true for all exergonic reactions?
A) The products have more total energy than the reactants.
B) The reaction proceeds with a net release of free energy.
C) The reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.
D) A net input of energy from the surroundings is required for the reactions to proceed.
B) The reaction proceeds with a net release of free energy.
A chemical reaction that has a positive ΔG is best described as _____.
A) endergonic
B) enthalpic
C) spontaneous
D) exergonic
A) endergonic
Chemical equilibrium is relatively rare in living cells. An example of a reaction at chemical equilibrium in a cell would be _____.
A) one in which the free energy at equilibrium is higher than the energy content at any point away from equilibrium
B) one in which the entropy change in the reaction is just balanced by an opposite entropy change in the cell's surroundings
C) an endergonic reaction in an active metabolic pathway where the energy for that reaction is supplied only by heat from the environment
D) a chemical reaction in which both the reactants and products are not being produced or used in any active metabolic pathway at that time in the cell
D) a chemical reaction in which both the reactants and products are not being produced or used in any active metabolic pathway at that time in the cell
Choose the pair of terms that correctly completes this sentence: Catabolism is to anabolism as _____ is to _____.
A) exergonic; spontaneous
B) exergonic; endergonic
C) free energy; entropy
D) work; energy
B) exergonic; endergonic
In solution, why do hydrolysis reactions occur more readily than condensation reactions?
A) Hydrolysis increases entropy and is exergonic.
B) Hydrolysis raises G, or Gibbs free energy.
C) Hydrolysis decreases entropy and is exergonic.
D) Hydrolysis increases entropy and is endergonic.
A) Hydrolysis increases entropy and is exergonic.
Why is ATP an important molecule in metabolism?
A) Its hydrolysis provides an input of free energy for exergonic reactions.
B) It provides energy coupling between exergonic and endergonic reactions.
C) Its terminal phosphate group contains a strong covalent bond that, when hydrolyzed, releases free energy.
D) Its terminal phosphate bond has higher energy than the other two phosphate bonds.
B) It provides energy coupling between exergonic and endergonic reactions.
When 10,000 molecules of ATP are hydrolyzed to ADP and i in a test tube, about half as much heat is liberated as when a cell hydrolyzes the same amount of ATP. Which of the following is the best explanation for this observation?
A) Cells are open systems, but a test tube is an isolated system.
B) Cells are less efficient at heat production than nonliving systems.
C) The reaction in cells must be catalyzed by enzymes, but the reaction in a test tube does not need enzymes.
D) Reactant and product concentrations in the test tube are different from those in the cell.
D) Reactant and product concentrations in the test tube are different from those in the cell.
Which of the following is most similar in structure to ATP?
A) a pentose sugar
B) a DNA nucleotide
C) an RNA nucleotide
D) an amino acid with three phosphate groups attached
C) an RNA nucleotide
Catabolic pathways _____.
A) combine molecules into more energy-rich molecules
B) supply energy, primarily in the form of ATP, for the cell's work
C) are endergonic
D) are spontaneous and do not need enzyme catalysis
B) supply energy, primarily in the form of ATP, for the cell's work
When chemical, transport, or mechanical work is done by an organism, what happens to the heat generated?
A) It is used to power yet more cellular work.
B) It is used to store energy as more ATP.
C) It is used to generate ADP from nucleotide precursors.
D) It is lost to the environment.
D) It is lost to the environment.
When ATP releases some energy, it also releases inorganic phosphate. What happens to the inorganic phosphate in the cell?
A) It is secreted as waste.
B) It is used only to regenerate more ATP.
C) It may be used to form a phosphorylated intermediate.
D) It enters the nucleus and affects gene expression.
C) It may be used to form a phosphorylated intermediate.
A number of systems for pumping ions across membranes are powered by ATP. Such ATP-powered pumps are often called ATPases, although they do not often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cytosolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system called the sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a free calcium ion concentration of 10-7 while the concentration in the SR is 10-2, then how is the ATPase acting?
A) ATPase activity must be powering an inflow of calcium from the outside of the cell into the SR.
B) ATPase activity must be transferring i to the SR to enable this to occur.
C) ATPase activity must be pumping calcium from the cytosol to the SR against the concentration gradient.
D) ATPase activity must be opening a channel for the calcium ions to diffuse back into the SR along the concentration gradient.
C) ATPase activity must be pumping calcium from the cytosol to the SR against the concentration gradient.
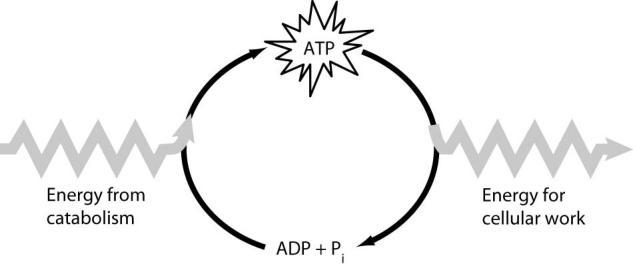
Which of the following is the most correct interpretation of the figure?
A) Energy from catabolism can be used directly for performing cellular work.
B) ADP + i are a set of molecules that store energy for catabolism.
C) ATP is a molecule that acts as an intermediary to store energy for cellular work.
D) i acts as a shuttle molecule to move energy from ATP to ADP.
C) ATP is a molecule that acts as an intermediary to store energy for cellular work.
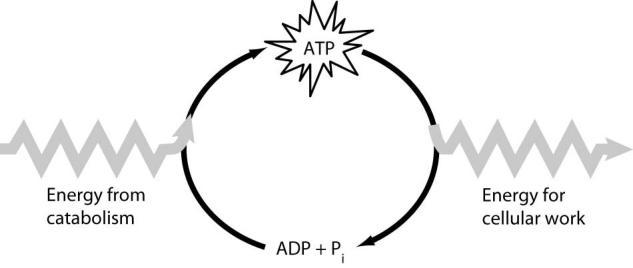
How do cells use the ATP cycle shown in the figure?
A) Cells use the cycle to recycle ADP and phosphate.
B) Cells use the cycle to recycle energy released by ATP hydrolysis.
C) Cells use the cycle to recycle ADP, phosphate, and the energy released by ATP hydrolysis.
D) Cells use the cycle primarily to generate heat.
- A) Cells use the cycle to recycle ADP and phosphate.
Which of the following is true of enzymes?
A) Enzyme function is increased if the 3- D structure or conformation of an enzyme is altered.
B) Enzyme function is independent of physical and chemical environmental factors such as pH and temperature.
C) Enzymes increase the rate of chemical reaction by lowering activation energy barriers.
D) Enzymes increase the rate of chemical reaction by providing activation energy to the substrate.
C) Enzymes increase the rate of chemical reaction by lowering activation energy barriers.
Which of the following is true when comparing an uncatalyzed reaction to the same reaction with a catalyst?
A) The catalyzed reaction will be slower.
B) The catalyzed reaction will have the same ΔG.
C) The catalyzed reaction will have higher activation energy.
D) The catalyzed reaction will consume all of the catalyst.
B) The catalyzed reaction will have the same ΔG.
The lock-and-key analogy for enzymes applies to the specificity of enzymes _____.
A) as they form their tertiary and quaternary structure
B) binding to their substrate
C) interacting with water
D) interacting with ions
B) binding to their substrate
You have discovered an enzyme that can catalyze two different chemical reactions. Which of the following is most likely to be correct?
A) The enzyme contains α-helices and β-pleated sheets.
B) The enzyme is subject to competitive inhibition and allosteric regulation.
C) Two types of allosteric regulation occur: The binding of one molecule activates the enzyme, while the binding of a different molecule inhibits it.
D) Either the enzyme has two distinct active sites or the reactants involved in the two reactions are very similar in size and shape.
D) Either the enzyme has two distinct active sites or the reactants involved in the two reactions are very similar in size and shape.
Reactants capable of interacting to form products in a chemical reaction must first overcome a thermodynamic barrier known as the reaction's _____.
A) entropy
B) activation energy
C) equilibrium point
D) free-energy content
B) activation energy
During a laboratory experiment, you discover that an enzyme-catalyzed reaction has a ΔG of -20 kcal/mol. If you double the amount of enzyme in the reaction, what will be the ΔG for the new reaction?
A) -40 kcal/mol
B) -20 kcal/mol
C) 0 kcal/mol
D) +20 kcal/mol
B) -20 kcal/mo
The active site of an enzyme is the region that _____.
A) binds allosteric regulators of the enzyme
B) is involved in the catalytic reaction of the enzyme
C) binds noncompetitive inhibitors of the enzyme
D) is inhibited by the presence of a coenzyme or a cofactor
B) is involved in the catalytic reaction of the enzyme
According to the induced fit hypothesis of enzyme catalysis, _____.
A) the binding of the substrate depends on the shape of the active site
B) some enzymes change their structure when activators bind to the enzyme
C) the binding of the substrate changes the shape of the enzyme's active site
D) the active site creates a microenvironment ideal for the reaction
C) the binding of the substrate changes the shape of the enzyme's active site
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following?
A) the need for a coenzyme
B) allosteric inhibition
C) competitive inhibition
D) insufficient cofactors
C) competitive inhibition
) Zinc, an essential trace element for most organisms, is present in the active site of the enzyme carboxypeptidase. The zinc most likely functions as _____.
A) a noncompetitive inhibitor of the enzyme
B) an allosteric activator of the enzyme
C) a cofactor necessary for enzyme activity
D) a coenzyme derived from a vitamin
C) a cofactor necessary for enzyme activity
A noncompetitive inhibitor decreases the rate of an enzyme reaction by _____.
A) binding at the active site of the enzyme
B) changing the shape of the enzyme's active site
C) changing the free energy change of the reaction
D) acting as a coenzyme for the reaction
B) changing the shape of the enzyme's active site
You collect data on the effect of pH on the function of the enzyme catalase in human cells. Which of the following graphs would you expect?
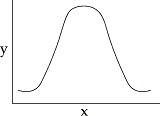
C
How might a change of one amino acid at a site, distant from the active site of an enzyme, alter an enzyme's substrate specificity?
A) by changing the enzyme's stability
B) by changing the shape of an enzyme
C) by changing the enzyme's pH optimum
D) An amino acid change away from the active site cannot alter the enzyme's substrate specificity.
B) by changing the shape of an enzyme
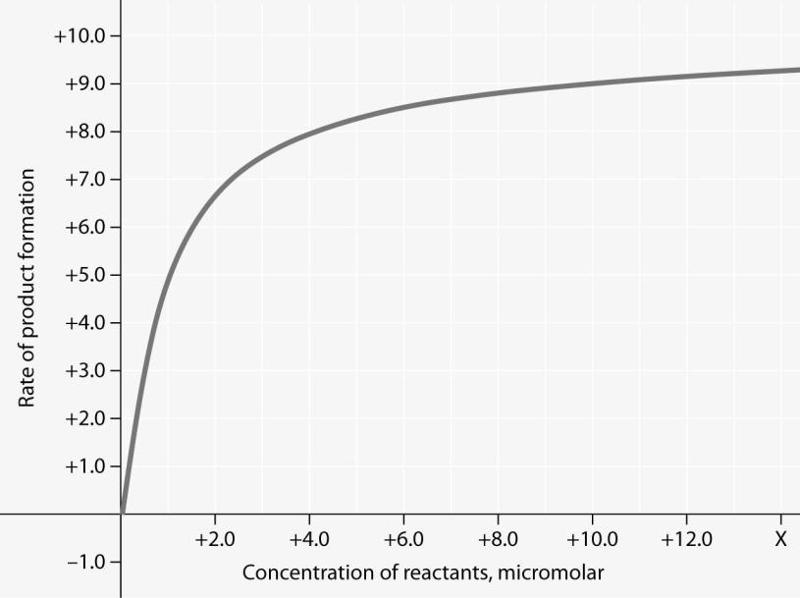
Rate of an enzyme-catalyzed reaction as a function of varying reactant concentration, with the concentration of enzyme constant.
For the enzyme- catalyzed reaction shown in the figure, if the initial reactant concentration is 1.0 micromolar, which of these treatments will cause the greatest increase in the rate of the reaction?
A) doubling the activation energy needed
B) cooling the reaction by 10°C
C) doubling the enzyme concentration
D) increasing the concentration of reactants to 10.0 micromolar, while reducing the concentration of enzyme by 1/2
C) doubling the enzyme concentration
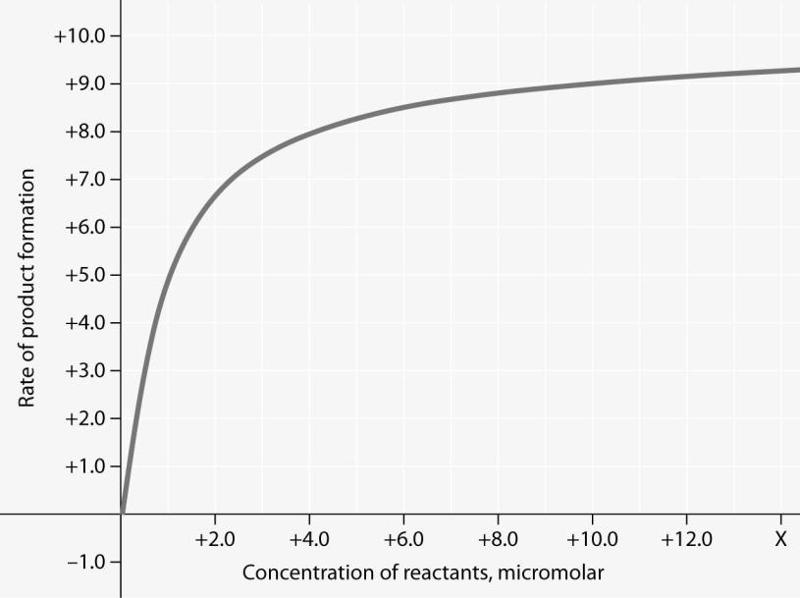
Rate of an enzyme-catalyzed reaction as a function of varying reactant concentration, with the concentration of enzyme constant.
In the figure, why does the reaction rate plateau at higher reactant concentrations?
A) Feedback inhibition by product occurs at high reactant concentrations.
B) Most enzyme molecules are occupied by substrate at high reactant concentrations.
C) The reaction nears equilibrium at high reactant concentrations.
D) The rate of the reverse reaction increases with reactant concentration.
B) Most enzyme molecules are occupied by substrate at high reactant concentrations.
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
Based on this information, which of the following is correct?
A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate.
B) Succinate dehydrogenase is the enzyme, and malonic acid is the substrate.
C) Succinate is the substrate, and fumarate is the product.
D) Fumarate is the product, and malonic acid is a noncompetitive inhibitor.
C) Succinate is the substrate, and fumarate is the product.
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
What is malonic acid's role with respect to succinate dehydrogenase? Malonic acid _____.
A) is a competitive inhibitor
B) blocks the binding of fumarate
C) is a noncompetitive inhibitor
D) is an allosteric regulator
A) is a competitive inhibitor
HIV is the virus that causes AIDS. In the mid-1990s, researchers discovered an enzyme in HIV called protease. Once the enzyme's structure was known, researchers began looking for drugs that would fit into the active site and block it. If this strategy for stopping HIV infections were successful, it would be an example of what phenomenon?
A) vaccination
B) denaturation
C) allosteric regulation
D) competitive inhibition
D) competitive inhibition
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
What is substance X?
A) an allosteric inhibitor
B) a substrate
C) an intermediate
D) the product
B) a substrate
A series of enzymes catalyze the reaction X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme.
With respect to the enzyme that converts X to Y, substance A functions as _____.
A) an allosteric inhibitor
B) the substrate
C) an intermediate
D) a competitive inhibitor
A) an allosteric inhibitor
The mechanism in which the end product of a metabolic pathway inhibits an earlier step in the pathway is most precisely described as _____.
A) metabolic inhibition
B) feedback inhibition
C) allosteric inhibition
D) noncooperative inhibition
B) feedback inhibition
You have isolated a previously unstudied protein, identified its complete structure in detail, and determined that it catalyzes the breakdown of a large substrate. You notice it has two binding sites. One of these is large, apparently the bonding site for the large substrate; the other is small, possibly a binding site for a regulatory molecule. What do these findings tell you about the mechanism of this protein?
A) It is probably a structural protein that is involved in cell-to-cell adhesion.
B) It is probably an enzyme that works through allosteric regulation.
C) It is probably an enzyme that works through competitive inhibition.
D) It is probably a cell membrane transport protein–like an ion channel.
B) It is probably an enzyme that works through allosteric regulation.
Allosteric enzyme regulation is usually associated with _____.
A) feedback inhibition
B) activating activity
C) an enzyme with more than one subunit
D) the need for cofactors
C) an enzyme with more than one subunit
Which of the following is an example of cooperativity?
A) the binding of an end product of a metabolic pathway to the first enzyme that acts in the pathway
B) one enzyme in a metabolic pathway passing its product to act as a substrate for the next enzyme in the pathway
C) a molecule binding at one unit of a tetramer, allowing faster binding at each of the other three
D) binding of an ATP molecule along with one of the substrate molecules in an active site
C) a molecule binding at one unit of a tetramer, allowing faster binding at each of the other three
Besides turning enzymes on or off, what other means does a cell use to control enzymatic activity?
A) localization of enzymes into specific organelles or membranes
B) exporting enzymes out of the cell
C) connecting enzymes into large aggregates
D) hydrophobic interactions
A) localization of enzymes into specific organelles or membranes
Protein kinases are enzymes that transfer the terminal phosphate from ATP to an amino acid residue on the target protein. Many are located on the plasma membrane as integral membrane proteins or peripheral membrane proteins. What purpose may be served by their plasma membrane localization?
A) ATP is more abundant near the plasma membrane.
B) They can more readily encounter and phosphorylate other membrane proteins.
C) Membrane localization lowers the activation energy of the phosphorylation reaction.
D) They flip back and forth across the membrane to access target proteins on either side.
B) They can more readily encounter and phosphorylate other membrane proteins.
Biological systems use free energy based on empirical data that all organisms require a constant energy input. The first law of thermodynamics states that energy can be neither created nor destroyed. For living organisms, which of the following statements is an important consequence of this first law?
A) The energy content of an organism is constant except for when its cells are dividing.
B) The organism must ultimately obtain all the necessary energy for life from its environment.
C) The entropy of an organism decreases with time as the organism grows in complexity.
D) Organisms are unable to transform energy from the different states in which it can exist.
B) The organism must ultimately obtain all the necessary energy for life from its environment.
In a biological reaction, succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, a substance that resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the amount of succinate molecules to those of malonic acid reduces the inhibitory effect if malonic acid. Select the correct identification of the molecules described in the reaction.
A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate in the reaction.
B) Succinate dehydrogenase is the enzyme, and malonic acid is the substrate in the reaction.
C) Succinate is the substrate, and fumarate is the product in the reaction.
D) Fumarate is the product, and malonic acid is a noncompetitive inhibitor in the reaction.
C) Succinate is the substrate, and fumarate is the product in the reaction.