What factors are most important in determining which elements are most common in living matter?
- E) both the relative abundances of the elements and the emergent properties of the compounds made from these elements
Why is each element unique and different from other elements in chemical properties?
C) Each element has a unique number of protons in its nucleus.
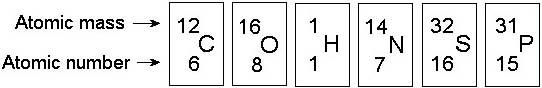
Knowing just the atomic mass of an element allows inferences about which of the following?
D) the number of protons plus neutrons in the element
Electrons exist only at fixed levels of potential energy. However, if an atom absorbs sufficient energy, a possible result is that
A) an electron may move to an electron shell farther away from the nucleus.
Which of the following explains most specifically the attraction of water molecules to one another?
D) hydrogen bond
In the term trace element, the modifier trace means that
A) the element is required in very small amounts.
Van der Waals interactions result when
B) electrons are not symmetrically distributed in a molecule.
Which of the following correctly describes chemical equilibrium?
A) Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
In the figure above, how many electrons does nitrogen have in its valence shell?
B) 5
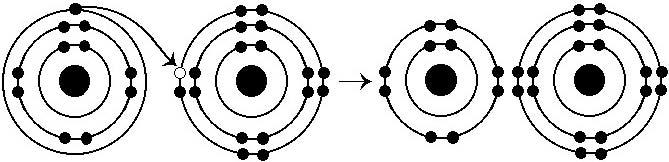
What results from the chemical reaction illustrated above?
E) a cation with a net charge of +1 and an anion with a net charge of -1
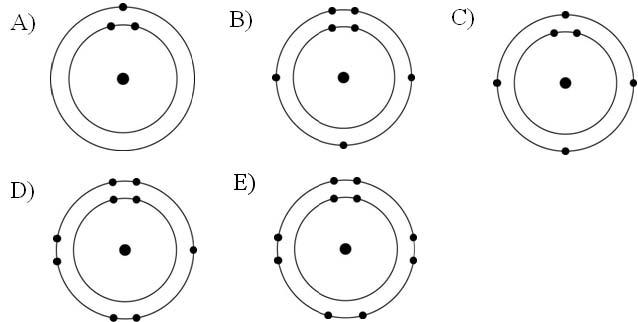
Which one of the atoms shown would be most likely to form an anion with a charge of -1?
Answer: D
Compared with ³¹P, the radioactive isotope ³²P has
E) one more neutron.
About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
D) carbon, hydrogen, nitrogen, oxygen
Liquid water's high specific heat is mainly a consequence of the
C) absorption and release of heat when hydrogen bonds break and form.
Which type of bond must be broken for water to vaporize?
D) hydrogen bonds
Why does ice float in liquid water?
D) Hydrogen bonds stabilize and keep the molecules of ice farther apart than the water molecules of liquid water.
You have a freshly prepared 0.1 M solution of glucose in water. Each liter of this solution contains how many glucose molecules?
E) 6.02 × 10²²
What is the pH of a solution with a hydroxyl ion [OH⁻] concentration of 10⁻¹² M?
A) pH 2
If the pH of a solution is decreased from 9 to 8, it means that the
E) concentration of H⁺ has increased tenfold (10X) and the concentration of OH⁻ has decreased to one-tenth (1/10) what they were at pH 9.
How would acidification of seawater affect marine organisms?
D) Acidification would decrease dissolved carbonate concentrations and hinder growth of corals and shell-building animals.
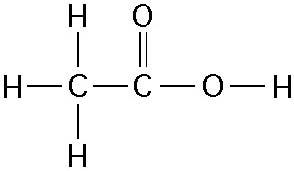
How many grams of the compound in the figure above would be required
to make 1 L of a 0.5 M solution?
(carbon = 12, oxygen = 16,
hydrogen = 1)
B) 30
The partial negative charge in a molecule of water occurs because
B) the electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom nucleus than around the hydrogen atom nucleus.
Which of the following effects is produced by the high surface tension of water?
B) A water strider can walk across the surface of a small pond.
Hydrophobic substances such as vegetable oil are
A) nonpolar substances that repel water molecules.
What is the hydrogen ion [H⁺] concentration of a solution of pH 8?
D) 10⁻⁸ M
If the pH of a solution is increased from pH 5 to pH 7, it means that the
C) concentration of OH⁻ is 100 times greater than what it was at pH 5.
The element present in all organic molecules is
C) carbon.
Hermann Kolbe's synthesis of an organic compound, acetic acid, from inorganic substances that had been prepared directly from pure elements was a significant milestone for what reason?
E) It proved that organic compounds could be synthesized from inorganic compounds and disproved the concept of vitalism.
Which of the following statements correctly describes cis-trans isomers?
A) They have variations in arrangement around a double bond.
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, a hydrocarbon chain with the same number of carbon atoms, but with one or more double bonds, will
B) be more constrained in structure.
Organic molecules with only hydrogens and five carbon atoms can have different structures in all of the following ways except
E) by forming enantiomers.
Which two functional groups are always found in amino acids?
C) carboxyl and amino
Testosterone and estradiol are
B) structural isomers of each other.
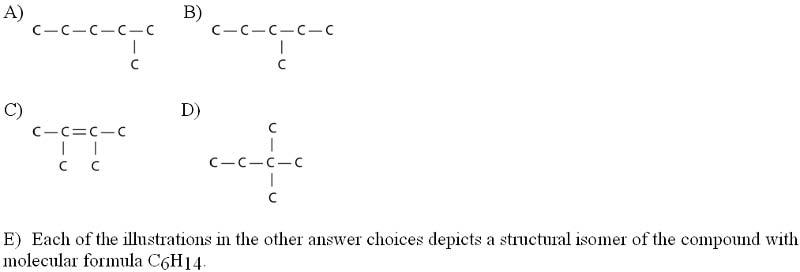
Three or four of the following illustrations depict different
structural isomers of the organic compound with molecular
formula
C6H14. For clarity, only the carbon skeletons are shown;
hydrogen atoms that would be attached to the carbons have
been
omitted. Which one, if any, is NOT a structural isomer of
this compound?
Answer: C
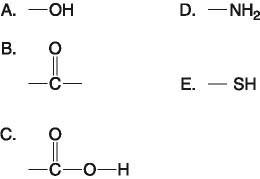
Which functional group(s) shown above is (are) present in all amino acids?
E) C and D
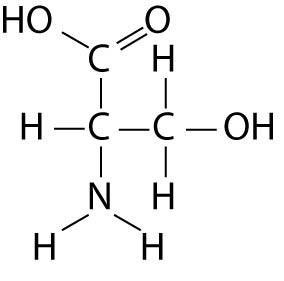
Which functional group is not present in this molecule?
B) sulfhydryl
Why are hydrocarbons insoluble in water?
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; that is, molecules that
B) are mirror images of one another.
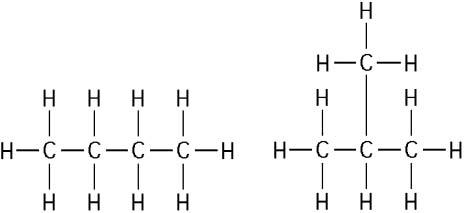
The two molecules shown in the figure above are best described as
C) structural isomers.
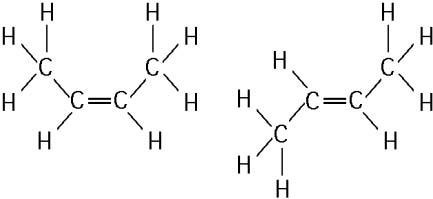
The two molecules shown in the figure above are best described as
E) cis-trans isomers.
Which of these molecules is not formed by dehydration reactions?
A) fatty acids
Which of the following is not a polymer?
A) glucose
On food packages, to what does the term insoluble fiber refer?
A) cellulose
All of the following contain amino acids except
B) cholesterol.
What aspects of protein structure are stabilized or assisted by hydrogen bonds?
E) secondary, tertiary, and quaternary structures, but not primary structure
Which bonds are created during the formation of the primary structure of a protein?
A) peptide bonds
What maintains the secondary structure of a protein?
B) hydrogen bonds between the amino group of one peptide bond and the carboxyl group of another peptide bond
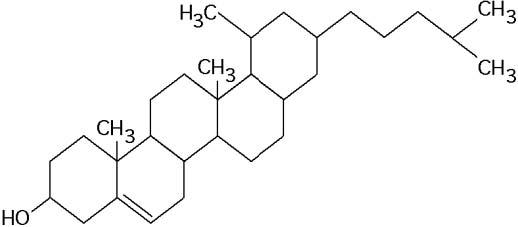
What is the structure shown in the figure below?
C) steroid molecule
Which class of biological polymer has the greatest functional variety?
b) proteins
The structural level of a protein least affected by a disruption in hydrogen bonding is the
A) primary level.
Which of the following statements is true for the class of biological molecules known as lipids?
A) They are insoluble in water.
There are 20 different amino acids. What makes one amino acid different from another?
C) different side chains (R groups) attached to an α carbon
Dehydration reactions are used in forming which of the following compounds?
E) triacylglycerides, polysaccharides, and proteins
The volume enclosed by the plasma membrane of plant cells is often much larger than the corresponding volume in animal cells. The most reasonable explanation for this observation is that
C) plant cells contain a large vacuole that reduces the volume of the cytoplasm.
Large numbers of ribosomes are present in cells that specialize in producing which of the following molecules?
C) proteins
A cell with a predominance of free ribosomes is most likely
B) producing primarily cytoplasmic proteins.
Hydrolytic enzymes must be segregated and packaged to prevent general destruction of cellular components. Which of the following organelles contains these hydrolytic enzymes in animal cells?
B) lysosome
One of the key innovations in the evolution of eukaryotes from a prokaryotic ancestor is the endomembrane system. What eukaryotic organelles or features might have evolved as a part of, or as an elaboration of, the endomembrane system?
D) nuclear envelope
If an individual has abnormal microtubules, due to a hereditary condition, in which organs or tissues would you expect dysfunction?
D) sperm, larynx, and trachea: cells and tissues that contain flagella or cilia
The cell walls of bacteria, fungi, and plant cells and the extracellular matrix of animal cells are all external to the plasma membrane. Which of the following is a characteristic common to all of these extracellular structures?
D) They are constructed of polymers that are synthesized in the cytoplasm and then transported out of the cell.
A mutation that disrupts the ability of an animal cell to add polysaccharide modifications to proteins would most likely cause defects in its
D) Golgi apparatus and extracellular matrix.
In a liver cell detoxifying alcohol and some other poisons, the enzymes of the peroxisome remove hydrogen from these molecules and
D) transfer the hydrogen to oxygen molecules to generate hydrogen peroxide.
The extracellular matrix is thought to participate in the regulation of animal cell behavior by communicating information from the outside to the inside of the cell via which of the following?
D) integrins
Plasmodesmata in plant cells are most similar in function to which of the following structures in animal cells?
C) gap junctions
What types of proteins are not synthesized in the rough ER?
D) mitochondrial proteins
Which type of organelle is found in plant cells but not in animal cells?
D) plastids
Which of the following is one of the ways that the membranes of winter wheat are able to remain fluid when it is extremely cold?
A) by increasing the percentage of unsaturated phospholipids in the membrane
Water passes quickly through cell membranes because
E) it moves through aquaporins in the membrane.
A bacterium engulfed by a white blood cell through phagocytosis will be digested by enzymes contained in
B) lysosomes.
In the small airways of the lung, a thin layer of liquid is needed between the epithelial cells and the mucus layer in order for cilia to beat and move the mucus and trapped particles out of the lung. One hypothesis is that the volume of this airway surface liquid is regulated osmotically by transport of sodium and chloride ions across the epithelial cell membrane. How would the lack of a functional chloride channel in cystic fibrosis patients affect sodium ion transport and the volume of the airway surface liquid?
C) Sodium ion transport will decrease; lower osmotic potential will decrease airway surface liquid volume.
A protein that spans the phospholipid bilayer one or more times is
A) a transmembrane protein.
What kinds of molecules pass through a cell membrane most easily?
B) small and hydrophobic
Which of the following would likely move through the lipid bilayer of a plasma membrane most rapidly?
A) CO₂
Which of the following statements is correct about diffusion?
C) It is a passive process in which molecules move from a region of higher concentration to a region of lower concentration.
Mammalian blood contains the equivalent of 0.15 M NaCl. Seawater contains the equivalent of 0.45 M NaCl. What will happen if red blood cells are transferred to seawater?
A) Water will leave the cells, causing them to shrivel and collapse.
When a plant cell, such as one from a peony stem, is submerged in a very hypotonic solution, what is likely to occur?
E) The cell will become turgid.
Glucose diffuses slowly through artificial phospholipid bilayers. The cells lining the small intestine, however, rapidly move large quantities of glucose from the glucose-rich food into their glucose-poor cytoplasm. Using this information, which transport mechanism is most probably functioning in the intestinal cells?
E) facilitated diffusion
The sodium-potassium pump is called an electrogenic pump because it
C) contributes to the membrane potential.
The movement of potassium into an animal cell requires
C) an energy source such as ATP.
Which term most precisely describes the cellular process of breaking down large molecules into smaller ones?
E) catabolism
Which of the following is (are) true for anabolic pathways?
C) They consume energy to build up polymers from monomers.
Which of the following is a statement of the first law of thermodynamics?
A) Energy cannot be created or destroyed.
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
D) As a consequence of growing, organisms cause a greater increase in entropy in their environment than the decrease in entropy associated with their growth.
Which of the following types of reactions would decrease the entropy within a cell?
A) anabolic reactions
The mathematical expression for the change in free energy of a system is ΔG =ΔH - TΔS. Which of the following is (are) correct?
C) ΔG is the change in free energy.
When ATP releases some energy, it also releases inorganic phosphate. What purpose does this serve (if any) in the cell?
D) The phosphate may be incorporated into any molecule that contains phosphate.
Reactants capable of interacting to form products in a chemical reaction must first overcome a thermodynamic barrier known as the reaction's
B) activation energy.
How does a noncompetitive inhibitor decrease the rate of an enzyme reaction?
B) by changing the shape of the enzyme's active site
Succinate dehydrogenase catalyzes the conversion of succinate to
fumarate. The reaction is inhibited by malonic acid, which resembles
succinate but cannot be acted upon by succinate dehydrogenase.
Increasing the ratio of succinate to malonic acid reduces the
inhibitory effect of malonic acid.
What is malonic
acid's role with respect to succinate dehydrogenase?
A) It is a competitive inhibitor.
If an enzyme in solution is saturated with substrate, the most effective way to obtain a faster yield of products is to
A) add more of the enzyme.
Which of the following is the smallest closed system?
E) the universe
A system at chemical equilibrium
E) can do no work.
Which of the following statements describes NAD⁺?
A) NAD⁺ is reduced to NADH during glycolysis, pyruvate oxidation, and the citric acid cycle.
Why are carbohydrates and fats considered high energy foods?
D) They have a lot of electrons associated with hydrogen.
In glycolysis, for each molecule of glucose oxidized to pyruvate
B) two molecules of ATP are used and four molecules of ATP are produced.
What is proton-motive force?
B) the force exerted on a proton by a transmembrane proton concentration gradient
In liver cells, the inner mitochondrial membranes are about five times the area of the outer mitochondrial membranes. What purpose must this serve?
C) It increases the surface for oxidative phosphorylation.
Which statement best supports the hypothesis that glycolysis is an ancient metabolic pathway that originated before the last universal common ancestor of life on Earth?
A) Glycolysis is widespread and is found in the domains Bacteria, Archaea, and Eukarya.
What is the purpose of beta oxidation in respiration?
E) breakdown of fatty acids
The immediate energy source that drives ATP synthesis by ATP synthase during oxidative phosphorylation is the
D) H⁺ concentration across the membrane holding ATP synthase.
Which metabolic pathway is common to both fermentation and cellular respiration of a glucose molecule?
C) glycolysis
The final electron acceptor of the electron transport chain that functions in aerobic oxidative phosphorylation is
A) oxygen.
When electrons flow along the electron transport chains of mitochondria, which of the following changes occurs?
A) The pH of the matrix increases.
Which process in eukaryotic cells will proceed normally whether oxygen (O₂) is present or absent?
B) glycolysis
A molecule that is phosphorylated
D) has an increased chemical potential energy; it is primed to do cellular work.
In any ecosystem, terrestrial or aquatic, what group(s) is (are) always necessary?
D) autotrophs
In autotrophic bacteria, where are the enzymes located that can carry on carbon fixation (reduction of carbon dioxide to carbohydrate)?
C) in the cytosol
A plant has a unique photosynthetic pigment. The leaves of this plant appear to be reddish yellow. What wavelengths of visible light are being absorbed by this pigment?
B) blue and violet
Assume a thylakoid is somehow punctured so that the interior of the thylakoid is no longer separated from the stroma. This damage will have the most direct effect on which of the following processes?
D) the synthesis of ATP
In photosynthetic cells, synthesis of ATP by the chemiosmotic mechanism occurs during
C) both photosynthesis and respiration.
In a plant leaf, the reactions that produce NADH occur in
D) neither the light reactions nor the Calvin cycle.
Which of the following statements best represents the relationships between the light reactions and the Calvin cycle?
A) The light reactions provide ATP and NADPH to the Calvin cycle, and the cycle returns ADP, Pi, and NADP⁺ to the light reactions.
A spaceship is designed to support animal life for a multiyear voyage
to the outer planets of the solar system. Plants will be grown to
provide oxygen and to recycle carbon dioxide.
Since the
spaceship will be too far from the sun for photosynthesis, an
artificial light source will be needed. What wavelengths of light
should be used to maximize plant growth with a minimum of energy expenditure?
C) a mixture of blue and red light
Where does the Calvin cycle take place?
A) stroma of the chloroplast
What are the products of linear photophosphorylation?
C) ATP and NADPH
Reduction of oxygen to form water occurs during
B) respiration only.
The reactions that produce molecular oxygen (O₂) take place in
A) the light reactions alone.
What is the primary function of the Calvin cycle?
E) synthesize simple sugars from carbon dioxide
The centromere is a region in which
A) chromatids remain attached to one another until anaphase.
Which of the following describe(s) cyclin-dependent kinase (Cdk)?
E) Cdk is present throughout the cell cycle and is an enzyme that attaches phosphate groups to other proteins.
Which of the following most accurately describes a cyclin?
D) It activates a Cdk molecule when it is in sufficient concentration.
Nucleotides can be radiolabeled before they are incorporated into
newly forming DNA and can therefore be assayed to track their
incorporation. In a set of experiments, a student—faculty research
team used labeled T nucleotides and introduced these into the culture
of dividing human cells at specific times.
Which of the
following questions might be answered by such a method?
B) What is the length of the S phase of the cell cycle?
Nucleotides can be radiolabeled before they are incorporated into newly forming DNA and can therefore be assayed to track their incorporation. In a set of experiments, a student—faculty research team used labeled T nucleotides and introduced these into the culture of dividing human cells at specific times.
The research team used the setup to study the incorporation of labeled nucleotides into a culture of lymphocytes and found that the lymphocytes incorporated the labeled nucleotide at a significantly higher level after a pathogen was introduced into the culture. They concluded that
C) infection causes lymphocytes to divide more rapidly.
One difference between cancer cells and normal cells is that cancer cells
C) continue to divide even when they are tightly packed together.
Which of the following does not occur during mitosis?
B) replication of the DNA
A particular cell has half as much DNA as some other cells in a mitotically active tissue. The cell in question is most likely in
A) G₁.
For anaphase to begin, which of the following must occur?
C) Cohesin must be cleaved enzymatically.
At the M phase checkpoint, the complex allows for what to occur?
A) Separase enzyme cleaves cohesins and allows chromatids to separate.
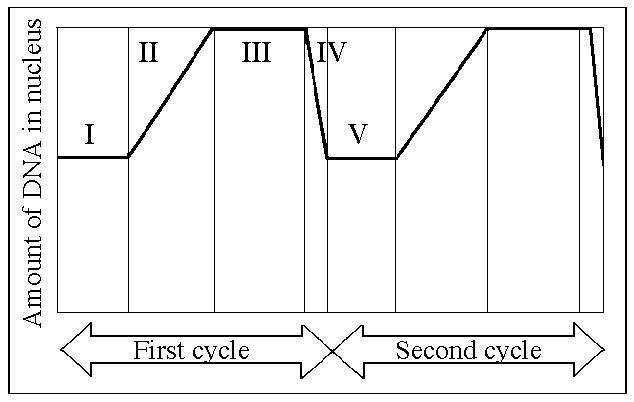
Which number represents DNA synthesis?
B) II
At which phase are centrioles beginning to move apart in animal cells?
E) prophase
Where do the microtubules of the spindle originate during mitosis in both plant and animal cells?
B) centrosome
In the human species, all somatic cells have 46 chromosomes. Which of the following can also be true?
A) A plant species (privet shrubs) has 46 chromosomes per cell.
The human X and Y chromosomes
D) include genes that determine an individual's sex.
Which of the following is true of a species that has a chromosome number of 2n = 16?
C) Each cell has eight homologous pairs.
Referring to a plant's sexual life cycle, which of the following terms describes the process that leads directly to the formation of gametes?
B) gametophyte mitosis
Which of the following best describes a karyotype?
B) a display of each of the chromosomes of a single cell
In a human karyotype, chromosomes are arranged in 23 pairs. If we choose one of these pairs, such as pair 14, which of the following do the two chromosomes of the pair have in common?
C) Length, centromere position, staining pattern, and traits coded for by their genes.
A cell divides to produce two daughter cells that are genetically different.
B) The statement is true for meiosis I only.
Independent assortment of chromosomes occurs.
B) The statement is true for meiosis I only.
A tetrad includes which of the following sets of DNA strands?
B) two sets of sister chromatids that have synapsed
To visualize and identify meiotic cells at metaphase with a microscope, what would you look for?
E) tetrads all aligned at the cell's center
Tetrads of chromosomes are aligned at the equator of the spindle; alignment determines independent assortment.
II. Metaphase I VI. Metaphase II
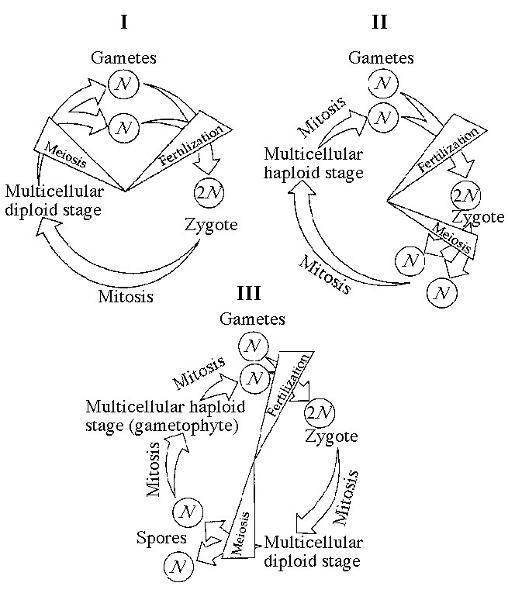
Which of the life cycles is typical for most fungi and some protists?
B) II only
After telophase I of meiosis, the chromosomal makeup of each daughter cell is
D) haploid, and the chromosomes are each composed of two chromatids.
Why did Mendel continue some of his experiments to the F₂ or F₃ generation?
B) to observe whether or not a recessive trait would reappear
Cystic fibrosis affects the lungs, the pancreas, the digestive system, and other organs, resulting in symptoms ranging from breathing difficulties to recurrent infections. Which of the following terms best describes this?
C) pleiotropy
Hydrangea plants of the same genotype are planted in a large flower garden. Some of the plants produce blue flowers and others pink flowers. This can be best explained by which of the following?
E) environmental factors such as soil pH
Which of the following provides an example of epistasis?
C) In rabbits and many other mammals, one genotype (cc) prevents any fur color from developing.
How could you best predict the maximum number of alleles for a single gene whose polypeptide product is known?
E) Count the number of DNA nucleotides that are in the code for the polypeptides.
One of two major forms of a human condition called neurofibromatosis (NF 1) is inherited as a dominant gene, although it may range from mildly to very severely expressed. If a young child is the first in her family to be diagnosed, which of the following is the best explanation?
B) One of the parents has very mild expression of the gene.
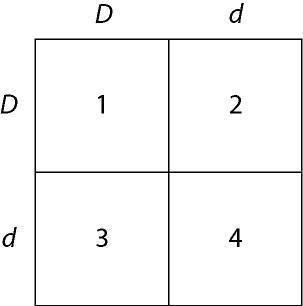
In a particular plant, leaf color is controlled by gene locus D. Plants with at least one allele D have dark green leaves, and plants with the homozygous recessive dd genotype have light green leaves. A true-breeding dark-leaved plant is crossed with a light-leaved one, and the F₁ offspring is allowed to self-pollinate. The predicted outcome of the F₂ is diagrammed in the Punnett square shown in Figure 14.1, where 1, 2, 3, and 4 represent the genotypes corresponding to each box within the square.
Which of the boxes marked 1-4 correspond to plants with dark leaves?
E) 1, 2, and 3
Two true-breeding stocks of pea plants are crossed. One parent has
red, axial flowers and the other has white, terminal flowers; all F₁
individuals have red, axial flowers. The genes for flower color and
location assort independently.
If 1,000 F₂ offspring resulted
from the cross, approximately how many of them would you expect to
have red, terminal flowers?
B) 190
Two true-breeding stocks of pea plants are crossed. One parent has
red, axial flowers and the other has white, terminal flowers; all F₁
individuals have red, axial flowers. The genes for flower color and
location assort independently.
Among the F₂ offspring, what is
the probability of plants with white axial flowers?
C) 3/16
Marfan syndrome in humans is caused by an abnormality of the connective tissue protein fibrillin. Patients are usually very tall and thin, with long spindly fingers, curvature of the spine, sometimes weakened arterial walls, and sometimes ocular problems, such as lens dislocation. Which of the following would you conclude about Marfan syndrome from this information?
D) It is pleiotropic.
When crossing an organism that is homozygous recessive for a single trait with a heterozygote, what is the chance of producing an offspring with the homozygous recessive phenotype?
C) 50%
Which of the following describes the ability of a single gene to have multiple phenotypic effects?
C) pleiotropy
Which of the following is an example of polygenic inheritance?
E) skin pigmentation in humans
Which of the following is the meaning of the chromosome theory of inheritance as expressed in the early 20th century?
B) Mendelian genes are at specific loci on the chromosome and in turn segregate during meiosis.
Thomas Hunt Morgan's choice of Drosophila melanogaster has been
proven to be useful even today. Which of the following has/have
continued to make it a most useful species?
I. its four pairs
of chromosomes
II. a very large number of visible as well as
biochemically mutant phenotypes
III. easy and inexpensive
maintenance
IV. short generation time and large number of offspring
E) I, II, III, IV, and V
Calico cats are female because
B) a male inherits only one of the two X-linked genes controlling hair color.
Duchenne muscular dystrophy (DMD) is caused by a gene on the human X chromosome. The patients have muscles that weaken over time because they have absent or decreased dystrophin, a muscle protein. They rarely live past their 20s. How likely is it for a woman to have this condition?
D) Very rarely would a woman have this condition; the condition would be due to a chromosome error.
Which of the following statements is true of linkage?
A) The closer two genes are on a chromosome, the lower the probability that a crossover will occur between them.
Why does recombination between linked genes continue to occur?
C) New allele combinations are acted upon by natural selection.
Map units on a linkage map cannot be relied upon to calculate physical distances on a chromosome for which of the following reasons?
A) The frequency of crossing over varies along the length of the chromosome.
A phenotypically normal prospective couple seeks genetic counseling because the man knows that he has a translocation of a portion of his chromosome 4 that has been exchanged with a portion of his chromosome 12. Although he is normal because his translocation is balanced, he and his wife want to know the probability that his sperm will be abnormal. What is your prognosis regarding his sperm?
A) 1/4 will be normal, 1/4 will have the translocation, and 1/2 will have duplications and deletions.
Which of the following is true of aneuploidies in general?
B) 45 X is the only known human live-born monosomy.
Mitochondrial DNA is primarily involved in coding for proteins needed for electron transport. Therefore, in which body systems would you expect most mitochondrial gene mutations to be exhibited?
D) the nervous and muscular systems
Sex determination in mammals is due to the SRY region of the Y chromosome. An abnormality of this region could allow which of the following to have a male phenotype?
B) translocation of SRY to an autosome of a 46, XX individual
An inversion in a human chromosome often results in no demonstrable phenotypic effect in the individual. What else may occur?
B) Some abnormal gametes may be formed.
What is the source of the extra chromosome 21 in an individual with Down syndrome?
D) nondisjunction or translocation in either parent
Cytosine makes up 42% of the nucleotides in a sample of DNA from an organism. Approximately what percentage of the nucleotides in this sample will be thymine?
A) 8%
Which of the following can be determined directly from X-ray diffraction photographs of crystallized DNA?
A) the diameter of the helix
In an analysis of the nucleotide composition of DNA, which of the following will be found?
C) A + C = G + T
Replication in prokaryotes differs from replication in eukaryotes for which of the following reasons?
B) Prokaryotic chromosomes have a single origin of replication, whereas eukaryotic chromosomes have many.
Which enzyme catalyzes the elongation of a DNA strand in the 5' → 3' direction?
C) DNA polymerase III
Polytene chromosomes of Drosophila salivary glands each consist of multiple identical DNA strands that are aligned in parallel arrays. How could these arise?
B) replication without separation
To repair a thymine dimer by nucleotide excision repair, in which order do the necessary enzymes act?
E) endonuclease, DNA polymerase I, DNA ligase
Which of the following would you expect of a eukaryote lacking telomerase?
D) a reduction in chromosome length in gametes
Which of the enzymes synthesizes short segments of RNA?
V. primase
Which of the following statements describes chromatin?
C) Heterochromatin is highly condensed, whereas euchromatin is less compact.
What is the function of topoisomerase?
A) relieving strain in the DNA ahead of the replication fork
What is the role of DNA ligase in the elongation of the lagging strand during DNA replication?
C) It joins Okazaki fragments together.
Which of the enzymes removes the RNA nucleotides from the primer and adds equivalent DNA nucleotides to the 3' end of Okazaki fragments?
IV. DNA polymerase I
Which of the enzymes separates the DNA strands during replication?
I. helicase
Which of the enzymes covalently connects segments of DNA?
III. ligase
Which of the following sets of materials are required by both eukaryotes and prokaryotes for replication?
A) double-stranded DNA, four kinds of dNTPs, primers, origins