Chapter 2
1) About twenty-five of the ninety-two natural elements are known to be essential to life. Which four of these twenty-five elements make up approximately 96 percent of living matter?
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen,
phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen,
nitrogen, oxygen
Answer: D
2) Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by humans and other vertebrates, but not by other organisms such as bacteria or plants?
A) calcium
B) iodine
C) sodium
D) phosphorus
Answer: B
3) Which of the following statements is FALSE?
A) Carbon, hydrogen, oxygen, and nitrogen are the most abundant
elements of living matter. B) Some naturally occurring elements are
toxic to organisms.
C) All life requires the same essential
elements.
D) Iron is needed by all humans.
Answer: C
4) Which of the following are compounds?
A) H2O, O2, and CH4
B) H2O and O2
C) O2 and CH4
D) H2O and CH4, but not O2
Answer: D
5) Knowing the atomic mass of an element allows inferences about which of the following?
A) the number of electrons in the element
B) the number of
protons in the element
C) the number of protons plus neutrons in
the element
D) the number of protons plus electrons in the element
Answer: C
6) In what way are elements in the same column of the periodic table the same? They have the same number of _____.
A) protons
B) electrons when neutral
C) electrons in their valence shells when neutral D) electron shells when neutral
Answer: C
7) Molybdenum has an atomic number of 42. Several common isotopes exist, with mass numbers from 92-100. Therefore, which of the following can be true?
A) Molybdenum atoms can have between 50 and 58 neutrons.
B)
Molybdenum atoms can have between 50 and 58 protons.
C) Molybdenum atoms can have between 50 and 58 electrons. D) Isotopes of molybdenum have different numbers of electrons.
Answer: A
8) Carbon-12 is the most common isotope of carbon and has a mass number of 12. However, the average atomic mass of carbon found on a periodic table is slightly more than 12 daltons. Why?
A) The atomic mass does not include the mass of electrons.
B) Some carbon atoms in nature have an extra proton.
C) Some carbon atoms in nature have more neutrons.
D) Some
carbon atoms in nature have a different valence electron distribution.
Answer: C

9) Which of the following best describes the relationship between the atoms described below?
A) They are isomers.
B) They are isotopes.
C) They
contain 1 and 3 protons, respectively.
D) They each contain only
1 neutron.
Answer: B
10) The atomic number of nitrogen is 7. Nitrogen-15 has a greater mass number than nitrogen-14 because the atomic nucleus of nitrogen-15 contains _____.
A) 7 neutrons
B) 8 neutrons
C) 8 protons
D) 15 protons
Answer: B
11) From its atomic number of 15, it is possible to predict that the phosphorus atom has _____.
A) 5 neutrons, 5 protons, and 5 electrons
B) 15 neutrons and
15 protons
C) 8 electrons in its outermost electron shell
D) 15 protons and 15 electrons
Answer: D
12) Fluorine has an atomic number of 9. Which of the following would you do to a neutral fluorine atom to complete its valence shell?
A) add 1 electron
B) add 2 electrons
C) remove 1 electron
D) Nothing. If fluorine is neutral, it
has a complete valance shell.
Answer: A
13) Magnesium has an atomic number of 12. What is the most stable charge for a magnesium ion?
A) a +1 charge
B) a +2 charge
C) a -1 charge
D) a -2 charge
Answer: B
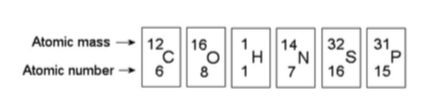
15) How many neutrons are present in the nucleus of a phosphorus-32 (32P) atom (see the figure above)?
A) 15
B) 16
C) 17
D) 32
Answer: C
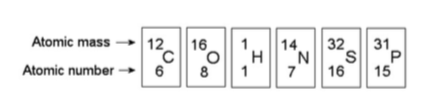
16) How many electrons will a single atom of sulfur with no charge
and no bonds have in its valence shell (see the figure above)?
A) 6
B) 8
C) 16
D) 32
Answer: A
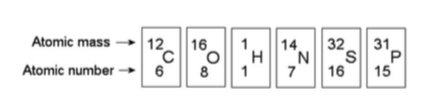
17) Based on electron configuration, which of the elements in the
figure above would exhibit a chemical behavior most like that of
oxygen?
A) carbon
B) nitrogen
C) sulfur
D) phosphorus
Answer: C

Answer: A

Answer: D
20) Oxygen has an atomic number of 8 and most commonly, a mass number
of 16. Thus, what is the atomic mass of an oxygen atom?
A)
approximately 8 grams
B) approximately 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
Answer: D
21) If you change the number of neutrons in an atom, you create _____.
A) a cation
B) an anion
C) an isotope
D) a different element
Answer: C
22) Can the atomic mass of an element vary?
A) No, it is fixed.
If it changes at all then you have formed a different element.
B) Yes. Adding or losing electrons will substantially change the
atomic mass.
C) Yes. Adding or losing protons will change the
atomic mass without forming a different element.
D) Yes. Adding
or losing neutrons will change the atomic mass without forming a
different element.
Answer: D
23) Which of the following is the best description of an atom's
physical structure?
A) An atom is a solid mass of
material.
B) The particles that form an atom are equidistant
from each other.
C) Atoms are little bubbles of space with mass
concentrated at the center of the bubble.
D) Atoms are little
bubbles of space with mass concentrated on the outside surface of the bubble.
Answer: C
24) A salamander relies on hydrogen bonding to stick to various
surfaces. Therefore, a salamander would have the greatest difficulty
clinging to a _____.
A) slightly damp surface
B) surface
of hydrocarbons
C) surface of mostly carbon-oxygen bonds D) surface of mostly carbon-nitrogen bonds
Answer: B
25) A covalent chemical bond is one in which _____.
A)
electrons are removed from one atom and transferred to another atom so
that the two atoms become oppositely charged
B) protons and
neutrons are shared by two atoms so as to satisfy the requirements of
both atoms
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill their respective orbitals
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom
Answer: C
26) What is the maximum number of covalent bonds that an oxygen atom
with atomic number 8 can make with hydrogen?
A) 1
B) 2
C) 4
D) 6
Answer: B
27) Nitrogen (N) is more electronegative than hydrogen (H). Which of
the following is a correct statement about the atoms in ammonia
(NH3)?
A) Each hydrogen atom has a partial positive charge; the
nitrogen atom has a partial negative charge.
B) Ammonia has an overall positive charge.
C) Ammonia has an
overall negative charge.
D) The nitrogen atom has a partial
positive charge; each hydrogen atom has a partial negative charge.
Answer: A
28) Bonds between two atoms that are equally electronegative are _____.
A) hydrogen bonds
B) polar covalent bonds
C) nonpolar
covalent bonds
D) ionic bonds
Answer: C
29) What results from an unequal sharing of electrons between atoms?
A) a nonpolar covalent bond
B) a polar covalent bond
C)
an ionic bond
D) a hydrophobic interaction
Answer: B
30) A covalent bond is likely to be polar when _____.
A) one of
the atoms sharing electrons is more electronegative than the other
atom B) the two atoms sharing electrons are equally
electronegative
C) carbon is one of the two atoms sharing
electrons
D) the two atoms sharing electrons are the same elements
Answer: A
31) What is the difference between covalent bonds and ionic
bonds?
A) Covalent bonds involve the sharing of pairs of
electrons between atoms; ionic bonds involve the sharing of single
electrons between atoms.
B) Covalent bonds involve the sharing
of electrons between atoms; ionic bonds involve the electrical
attraction between charged atoms.
C) Covalent bonds involve the
sharing of electrons between atoms; ionic bonds involve the sharing of
protons between charged atoms.
D) Covalent bonds involve the
transfer of electrons between charged atoms; ionic bonds involve the
sharing of electrons between atoms.
Answer: B
32) The atomic number of chlorine is 17. The atomic number of
magnesium is 12. What is the formula for magnesium chloride?
A)
MgCl
B) MgCl2
C) Mg2Cl
D) MgCl3
Answer: B
33) How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H4?
A) 1
B) 2
C) 3
D) 4
Answer: B
34) Which bond or interaction would be difficult to disrupt when compounds are put into water?
A) covalent bonds between carbon atoms
B) hydrogen
bonds
C) ionic bonds
D) ionic and hydrogen bonds
Answer: A
35) Water molecules are attracted to one another by _____.
A) nonpolar covalent bonds
B) ionic bonds
C) hydrogen bonds
D) hydrophobic interactions
Answer: C
36) Van der Waals interactions may result when _____.
A)
electrons are not symmetrically distributed in a molecule
B) molecules held by ionic bonds react with water
C) two
polar covalent bonds react
D) a hydrogen atom loses an electron
Answer: A
37) What is the maximum number of hydrogen atoms that can be
covalently bonded in a molecule containing two carbon atoms?
A)
2
B) 4
C) 6
D) 8
Answer: C
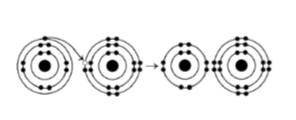
38) What results from the chemical reaction illustrated above? The reactants have no charge.
A) a cation with a net charge of +1 and an anion with a net charge
of +1
B) a cation with a net charge of -1 and an anion with a
net charge of -1
C) a cation with a net charge of -1 and an
anion with a net charge of +1
D) a cation with a net charge of +1 and an anion with a net charge of -1
Answer: D
39) What is the atomic number of the cation formed in the reaction illustrated above?
A) 8
B) 10
C) 11
D) 16
Answer: C
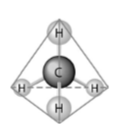
40) What causes the shape of the molecule shown above?
A) the shape of the 2 p orbitals in the carbon atom
B) the
shape of the 1 s orbital in the carbon atom
C) the shape of the sp3 hybrid orbitals of the electrons shared
between the carbon and hydrogen atoms
D) hydrogen bonding
configurations between the carbon and hydrogen atoms
Answer: C
41) How many electrons are involved in a single covalent bond?
A) one
B) two
C) three
D) four
Answer: B
42) How many electrons are involved in a double covalent bond?
A) one
B) two
C) three
D) four
Answer: D
43) If an atom has a charge of +1, which of the following must be true?
A) It has two more protons than neutrons.
B) It has the same
number of protons as electrons.
C) It has one more electron than
it does protons.
D) It has one more proton than it does electrons.
Answer: D
44) Elements found on the left side of the periodic table contain
outer shells that are _____; these elements tend to form _____ in
solution.
A) almost empty; cations
B) almost empty; anions
C) almost full; cations
D) almost full; anions
Answer: A
45) An atom has four electrons in its valence shell. What types of
covalent bonds is it capable of forming?
A) single, double, or
triple
B) single and double only
C) single bonds only
D) double bonds only
Answer: A
46) When are atoms most stable?
A) when they have the fewest
possible valence electrons
B) when they have the maximum number
of unpaired electrons
C) when all of the electron orbitals in the valence shell are filled
D) when all electrons are paired
Answer: C
47) When the atoms involved in a covalent bond have the same
electronegativity, what type of bond results?
A) an ionic
bond
B) a hydrogen bond
C) a nonpolar covalent bond
D) a polar covalent bond
Answer: C
48) Nitrogen (N) normally forms three covalent bonds with a valence
of 5. However, ammonium has four covalent bonds, each to a different
hydrogen (H) atom (H has a valence of 1). What do you predict to be
the charge on ammonium?
A) +1
B) -1
C) +2
D) -2
Answer: A
49) You need to write down information about a molecule, but need to
indicate only the type and number of atoms it contains. Which
representation would work best?
A) molecular formula
B)
structural formula
C) ball-and-stick model
D) space-filling model
Answer: A
50) You need to represent a molecule to best illustrate the relative
sizes of the atoms involved and their interrelationships. Which
representation would work best?
A) molecular formula
B)
structural formula
C) ball-and-stick model
D) space-filling model
Answer: D
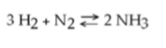
51) Which of the following is true for this reaction?
A) The reaction is nonreversible.
B) Hydrogen and nitrogen
are the reactants of the reverse reaction.
C) Ammonia is being formed and decomposed simultaneously.
D)
Only the forward or reverse reactions can occur at one time.
Answer: C
52) Which of the following correctly describes all chemical
equilibrium?
A) Forward and reverse reactions continue with no
net effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of
the reactants.
C) There are equal concentrations of products and
reactants while forward and reverse reactions continue.
D) There
are equal concentrations of reactants and products, and the reactions
have stopped.
Answer: A
53) Which of the following correctly describes a reaction that has reached chemical equilibrium?
A) The rate of the forward reaction is equal to the rate of the
reverse reaction.
B) All of the reactants have been converted to
the products of the reaction.
C) All of the products have been
converted to the reactants of the reaction.
D) Both the forward and the reverse reactions have stopped, with no net effect on the concentration of the reactants and the products.
Answer: A