What makes each element unique and different from other elements
Unique number of protons
What is the significance of atomic number
atomic mass?
Atomic number tells us the amount of protons in an atom
atomic mass tells us how many neutrons by subtracting the amount of protons
electrons can be found in the electron cloud. 1-2 On periodic table: electrons. 13-18 find by subtracting 10
What are isotopes
Variants of a particular element
different # of neutrons same # of protons
Atoms octet
If there are a certain number of electrons in an electron cloud you can either add or give away electrons to complete a perfect octet through sharing of electrons between elements
What are the max number of bonds specific elements can make?
Single H-H. Double bond= 2 pairs of electrons
double O= O. Triple bond= 3 pairs of electrons
triple N=_N
Define electro negativity
Level of charge given from one atom to attract another. Book Def: the attraction of a particular atom for the electrons of a covalent bond
How can electronegativity effect covalently bonded molecules
It takes partially greater charges from either positive and negatively bonded atoms and while shared equally, electrons will stay closer to the more electronegative charge
What happens if the electronegativity is equal
There is a standoff of tug of war and a non polar covalent bid is formed
What is the difference between ionic and covalent bonds?
Ionic bonds= electrons are not shared equally
covalent bonds= electrons are shared equally
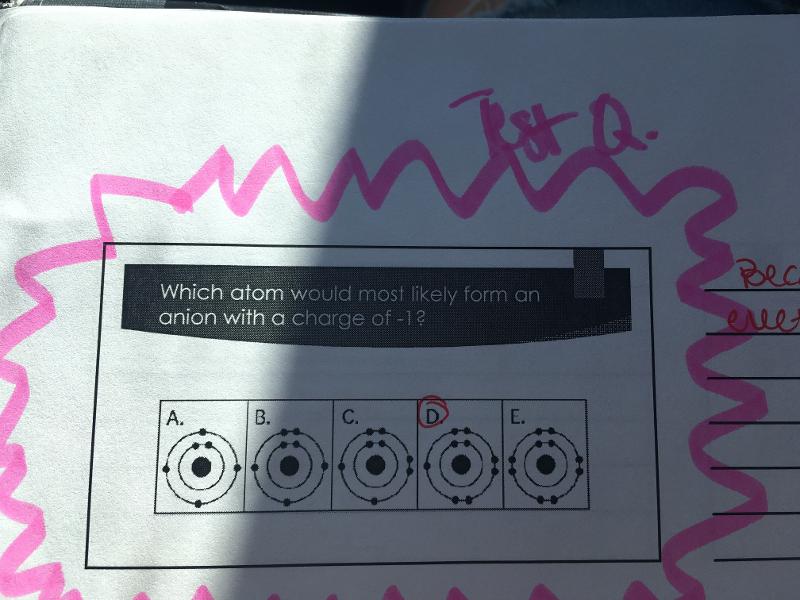
Because it will gain one electron
Structure of water - hydrogen bond, polar covalent bond, explain in detail partial negative charge of water molecule and why it occurs in terms of electronegativity and electrons
Positive H in one H2O molecule attracts to negative O in another. Polar covalent bond = partially negative/positive binding in a covalent bond and the electrons stay closer to the more electronegative side
Importance of hydrogen bonding to the properties of water
Hydrogen bonds form the basic structure of water for other bondings such as polar covalent to occur
Why do carbon atoms form diverse molecules
Carbon has 4 valence electrons do it can share electrons with other diverse molecules
Hydrophobic molecules and how they interact with water
Hydrophobic doesn’t dissolve in water. It is no polar and does not have an attraction to water
Interpret a PH scale
1-6 Acid 7 neutral 8-14 basic
The importance of buffers in biological systems, how do they balance PH?
They maintain a relative constant PH when either acids or bases are added to them
The role of dehydration reaction in organic compounds and hydrolysis in digestion of organic compounds
Dehydratiom reaction: assembles polymers releasing H2O
Hydrolysis: breaks down polymers consuming H2O
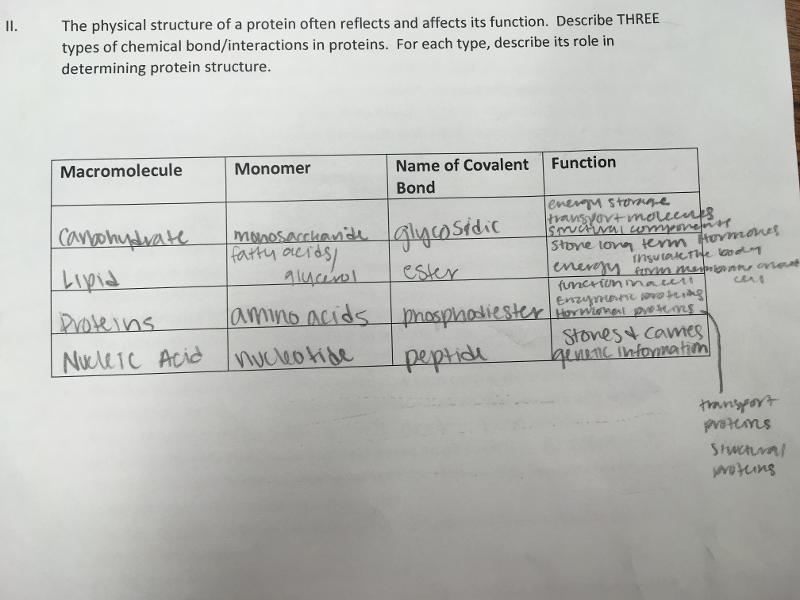
The four macromolecules and bonds that are formed durning the dehydration reaction
Carbohydrates: glycoscidic linkage
Lipid: ester linkage
nucleic acid: phosphodiester linkage
protein: peptide bond
Classify monosaccharides, disaccharides, and polysaccharides
Monosaccharid: monomer of carbohydrate ex: lactose glucose
Disaccharide: two monosaccharides ex: sucrose
polysaccharid: polymers ex: plants -store glucose as starch, animals -glycogen, cell structure in plants -cellulose
The difference between starch and cellulose and how it impacts digestion
Starch: stores energy in plants. Plants and animals can digest
cellulose: structural component in plants. CANNOT be digested by animals on account of beta glucose linkage
What’s the difference between saturated and unsaturated fats
Unsaturated: liquid, at least on double bond
saturated: solidify, contains more hydrogen,only single bonds, contributes to atherosclerosis (heart disease)
What is the first structural level of protein? How can it change the activity of the protein
Primary: A mess up will ruin all other structures. It dictates secondary and tertiary structure. Linear chain of amino acids
Second structural level of protein
Secondary: hydrogen bonds between an amino group of one peptide bond and the carbonyl group of another peptide bond
Third structural level of proteins
Tertiary: interaction between r groups. Disulfide bridge, ionic bond, hydrogen bond between at least one r group
Fourth structural level of proteins
Quaternary: two or more polypeptide chains into one large protein
What makes each amino group different from one another?
Each has a different R group
Name the bonds that are formed in primary and secondary structures of proteins
Primary: peptide
secondary: hydrogen
Identify the peptide bond if given a polypeptide:
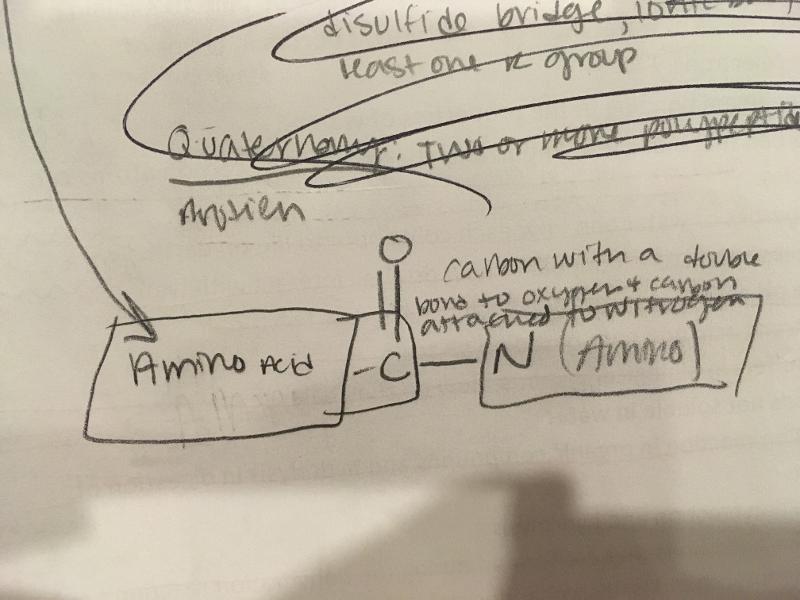
Carbon with a double bond to an oxygen single bonded to carbon attached to Nitrogen
What maintains structure of a protein
Hydrogen bonds
What is chaperonin
They help fold proteins within cells in the last structural level of proteins
Base pairing rules of DNA
A=T
C=G
Steroid structure
4 carbonn fused rings
Hydrocarbons are not soluble in water because
The C—H bond is nonpolar
What molecul a form a triacylglycerol/fat know and recognize the structure
G
l. Fatty acid 1
y
c
e. Fatty acid 2
r
o
L. Fatty acid 3
Bond between nucleotide to make nuclei acids - reaction forms it, type of bond
Phosphodiester covalent bond to form backbone from 5’ carbon and 3’ carbon
Recognize the the four biologically important organic compounds
Carbon
Hydrogen
Oxygen
Nitrogen
How do proteins reach their final shape
They go through all four phases and complete by using chaperonins