1) You have a planar bilayer with equal amounts of saturated and
unsaturated phospholipids. After testing the permeability of this
membrane to glucose, you increase the proportion of unsaturated
phospholipids in the bilayer. What will happen to the membrane's
permeability to glucose?
A) Permeability to glucose will
decrease.
B) Permeability to glucose will increase.
C)
Permeability to glucose will stay the same.
D) You cannot predict
the outcome. You simply have to make the measurement.
B
2) The membranes of winter wheat are able to remain fluid when it is
extremely cold by _____.
A) cotransport of glucose and
hydrogen
B) increasing the percentage of unsaturated
phospholipids in the membrane
C) decreasing the number of
hydrophobic proteins in the membrane
D) decreasing the percentage
of cholesterol molecules in the membrane
B
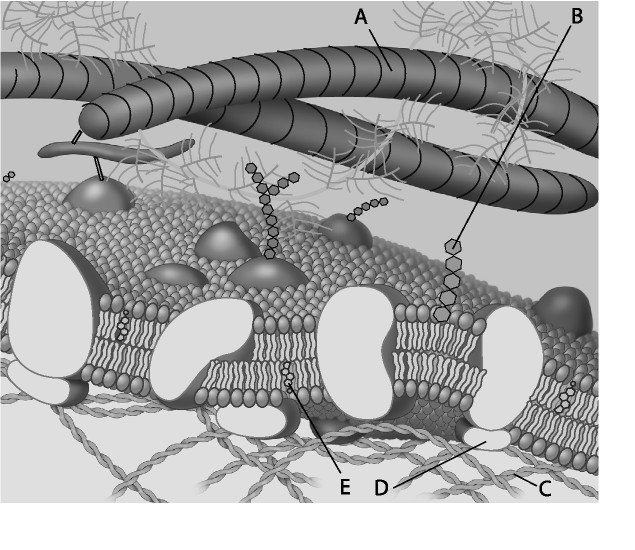
For the following questions, match the labeled component of the cell membrane in the figure with its description.
3) Which component is a peripheral protein?
A) A B) B C) C D) D
D
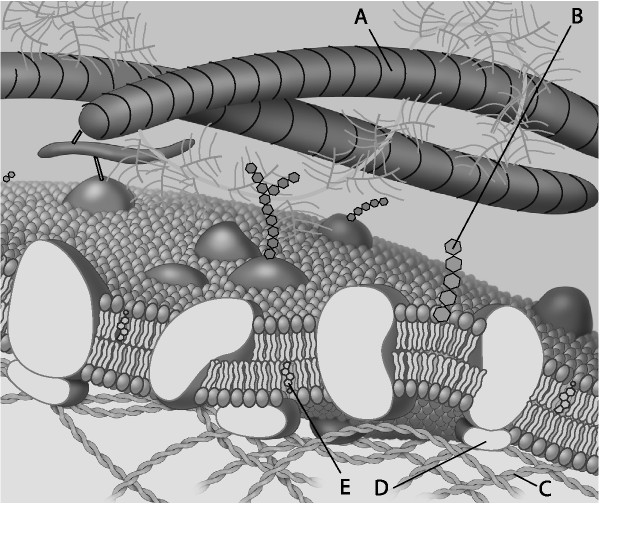
4) Which component is a protein fiber of the extracellular matrix?
A) A B) B C) C D) E
A
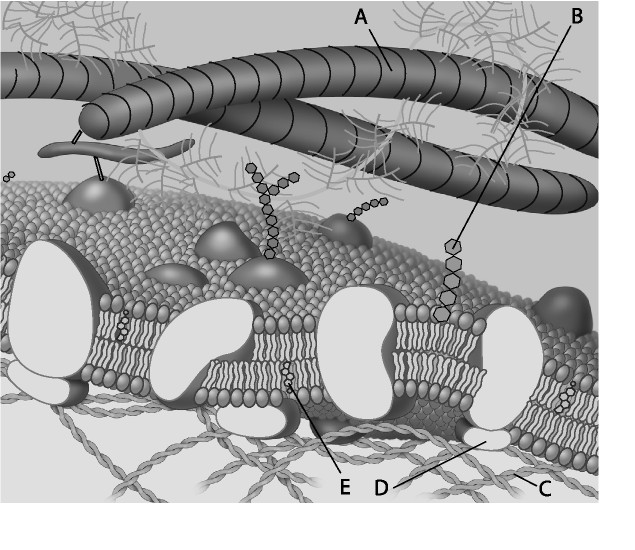
5) Which component is a microfilament (actin filament) of the cytoskeleton?
A) A B) B C) C D) D
C
6) Cell membranes are asymmetrical. Which of the following statements is the most likely explanation for the membrane's asymmetrical nature?
A) Proteins only function on the cytoplasmic side of the cell
membrane, which results in the membrane's asymmetrical nature.
B) The two sides of a cell membrane face different environments
and carry out different functions.
C) Since cell membranes
communicate signals from one organism to another, the cell membranes
must be asymmetrical.
D) Since the cell membrane forms a border
between one cell and another in tightly packed tissues such as
epithelium, the membrane must be asymmetrical
B
Succinate dehydrogenase catalyzes the conversion of succinate to fumarate. The reaction is inhibited by malonic acid, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate to malonic acid reduces the inhibitory effect of malonic acid.
7) What is malonic acid's role with respect to succinate
dehydrogenase? Malonic acid _____.
A) is a noncompetitive
inhibitor B) is a competitive inhibitor
C) blocks the binding of
fumarate D) is an allosteric regulator
B
8) Which of the following would likely move through the lipid bilayer
of a plasma membrane most rapidly?
A) an amino acid B) glucose
C) K+ D) CO2
D
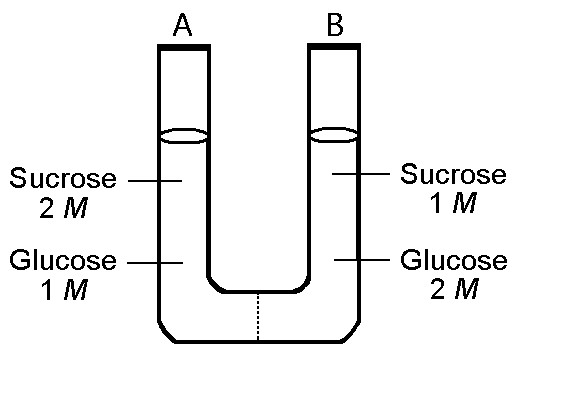
The solutions in the two arms of this U-tube are separated by a
membrane that is permeable to water and glucose but not to sucrose.
Side A is half-filled with a solution of 2 M sucrose and 1 M glucose.
Side B is half-filled with 1 M sucrose and 2 M glucose. Initially, the
liquid levels on both sides are equal.
9) Refer to the
figure. After the system reaches equilibrium, what changes are
observed?
A) The molarity of sucrose is higher than that of
glucose on side A.
B) The water level is higher in side B than in
side A.
C) The water level is higher in side A than in side
B.
D) The water level is unchanged.
C
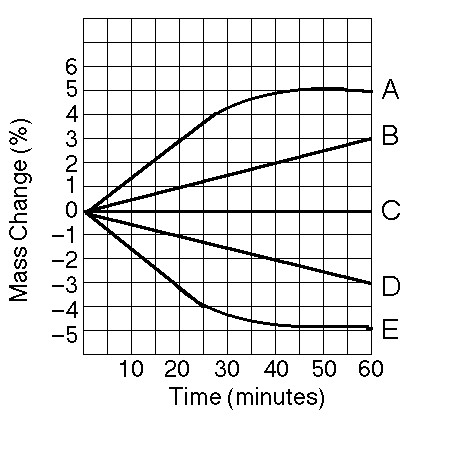
Five dialysis bags constructed of membrane, which is permeable to water and impermeable to sucrose, were filled with various concentrations of sucrose and then placed in separate beakers containing an initial concentration of 0.6 M sucrose solution. At 10-minute intervals, the bags were massed (weighed) and the percent change in mass of each bag was graphed.
10) Which line in the graph represents the bag with the highest initial concentration of sucrose?
A) A B) B C) C D) D
A
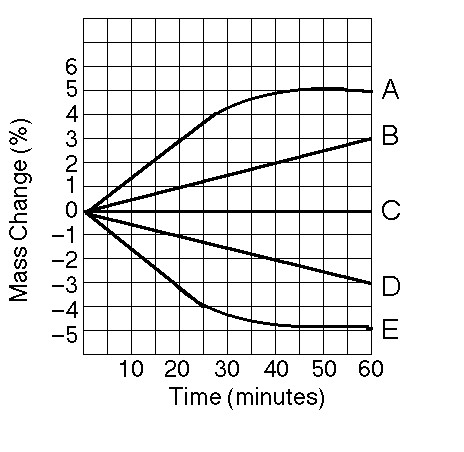
11) Which line or lines in the graph represent(s) bags that contain a
solution that is hypertonic at 50 minutes?
A) A and B B) B C) D
D) D and E
B
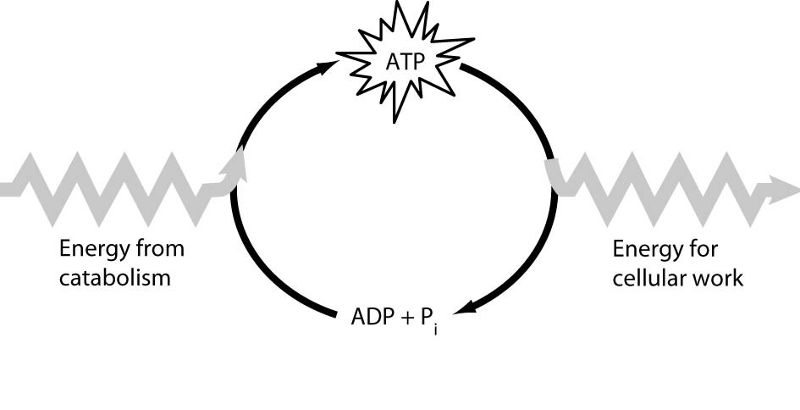
12) Which of the following is the most correct interpretation of the
figure?
A) ATP is a molecule that acts as an intermediary to
store energy for cellular work.
B) Energy from catabolism can be
used directly for performing cellular work.
C) ADP + i are a set
of molecules that store energy for catabolism.
D) i acts as a
shuttle molecule to move energy from ATP to ADP.
13) A system at
chemical equilibrium _____.
A) can do no work B) releases energy
at a steady rate
C) consumes energy at a steady rate D) has zero
kinetic energy
A
13) A system at chemical equilibrium _____.
A) can do no work
B) releases energy at a steady rate
C) consumes energy at a
steady rate D) has zero kinetic energy
A
14) You have discovered an enzyme that can catalyze two different
chemical reactions. Which of the following is most likely to be
correct?
A) Two types of allosteric regulation occur: The
binding of one molecule activates the enzyme, while the binding of a
different molecule inhibits it.
B) The enzyme contains α-helices
and β-pleated sheets.
C) Either the enzyme has two distinct
active sites or the reactants involved in the two reactions are very
similar in size and shape.
D) The enzyme is subject to
competitive inhibition and allosteric regulation.
C
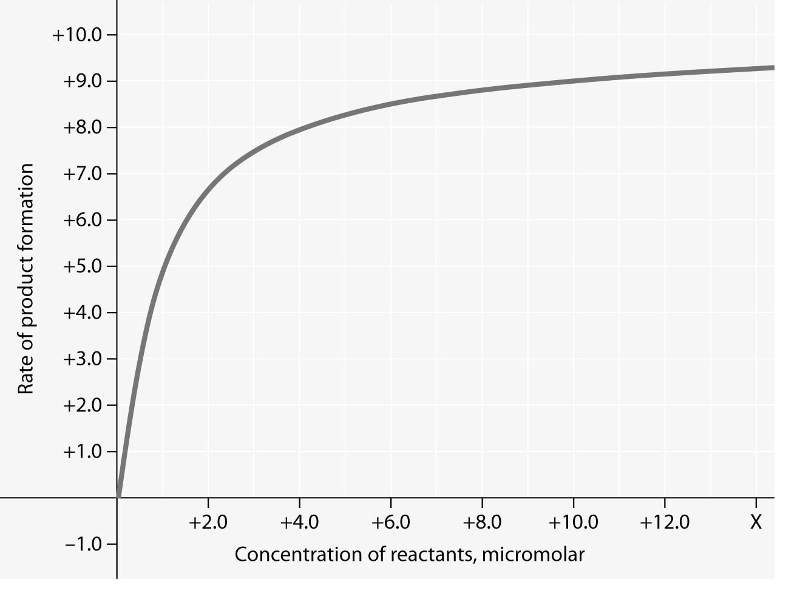
Rate of an enzyme-catalyzed reaction as a function of varying reactant concentration, with the concentration of enzyme constant.
15) In the figure, why does the reaction rate plateau at higher
reactant concentrations?
A) Feedback inhibition by product
occurs at high reactant concentrations.
B) The reaction nears
equilibrium at high reactant concentrations.
C) Most enzyme
molecules are occupied by substrate at high reactant
concentrations.
D) The rate of the reverse reaction increases
with reactant concentration.
C
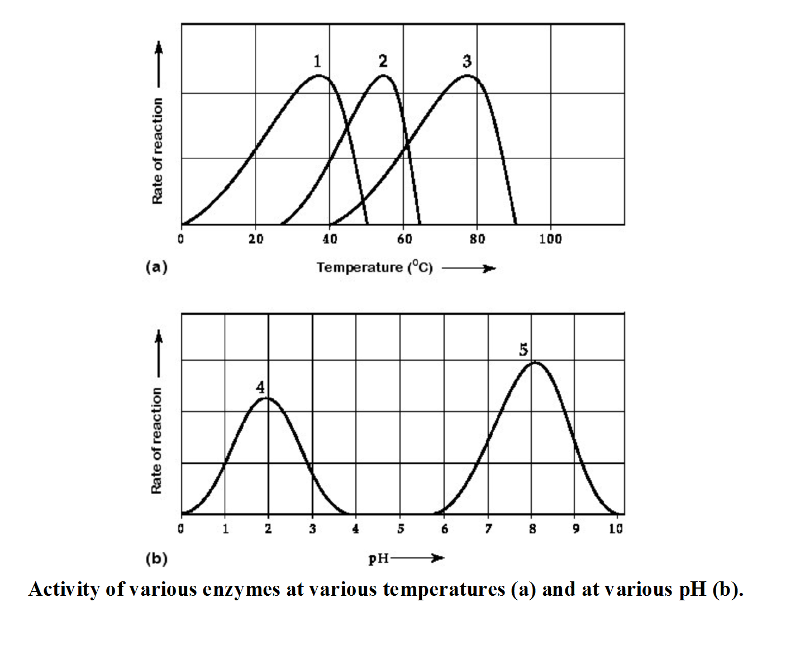
Activity of various enzymes at various temperatures (a) and at various pH (b).
16) Which curves on the graphs may represent the temperature and pH
profiles of an enzyme taken from a bacterium that lives in a mildly
alkaline hot springs at temperatures of 70°C or higher?
A)
curves 3 and 4 B) curves 1 and 5 C) curves 3 and 5 D) curves 2 and 5
C
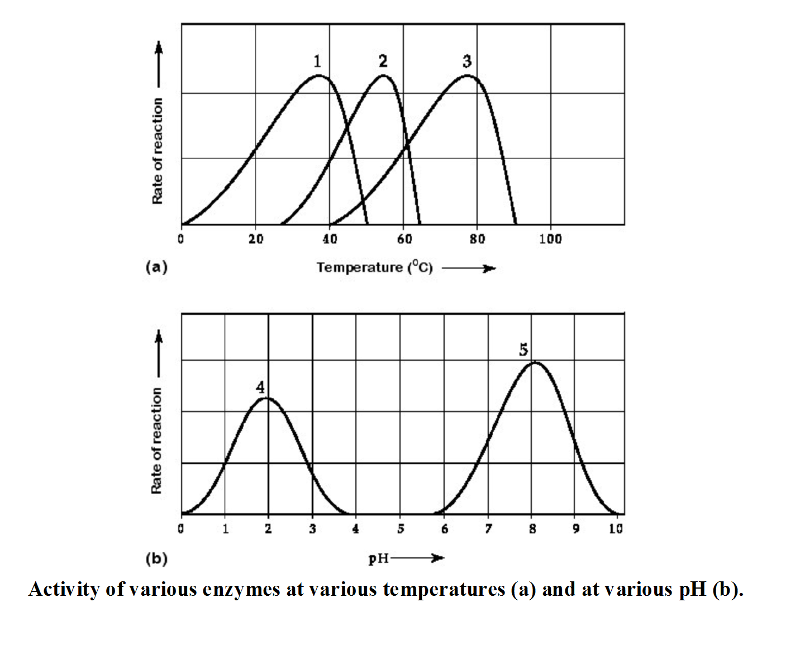
17) Which temperature and pH profile curves on the graphs were most
likely generated from analysis of an enzyme from a human stomach where
conditions are strongly acid?
A) curves 3 and 4 B) curves 1 and
5 C) curves 1 and 4 D) curves 2 and 4
C
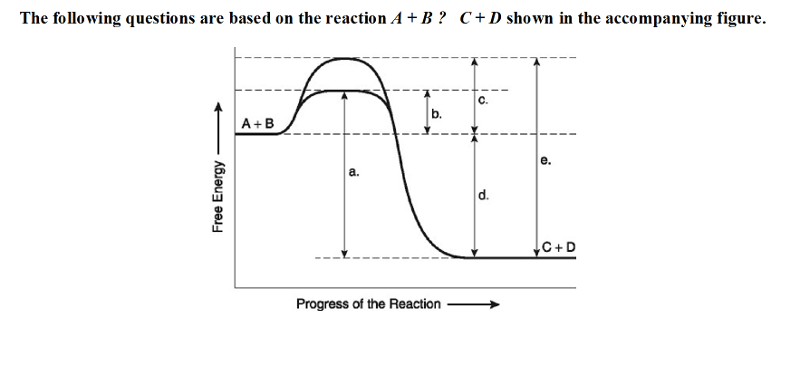
The following questions are based on the reaction A + B ↔ C + D shown in the accompanying figure.
18) Which of the following terms best describes the forward
reaction in the figure?
A) exergonic, ∆G < 0 B) exergonic,
∆G > 0
C) endergonic, ∆G < 0 D) endergonic, ∆G > 0
A
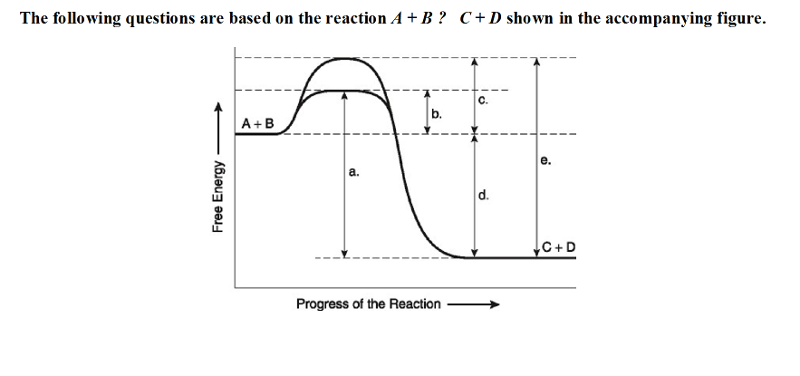
19) Which of the following in the figure would be the same in either
an enzyme-catalyzed or a noncatalyzed reaction?
A) a B) b C) c
D) d
D
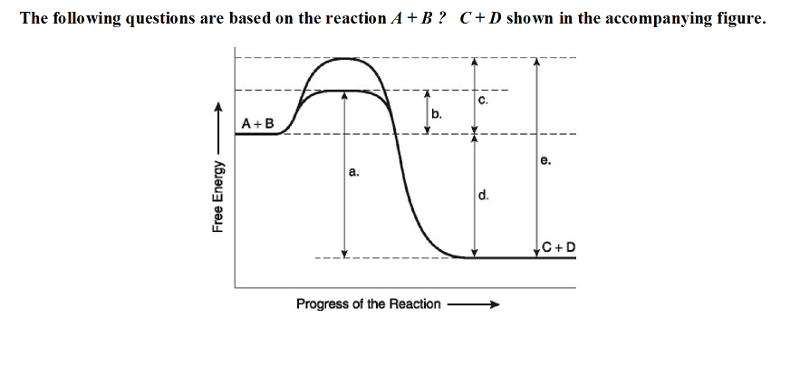
20) Which of the following represents the activation energy required
for the enzyme-catalyzed reaction in the figure?
A) a B) b C) c
D) d
B
21) Substrate-level phosphorylation accounts for approximately what percentage of the ATP formed by the reactions of glycolysis? A) 100% B) 38% C) 0% D) 2%
A
22) The chemiosmotic hypothesis is an important concept in our
understanding of cellular metabolism in general because it explains
A) the reduction of oxygen to water in the final steps of
oxidative metabolism
B) the sequence of the electron transport
chain molecules
C) how ATP is synthesized by a proton motive
force
D) how electron transport can fuel substrate-level phosphorylation
C
23) The synthesis of ATP by oxidative phosphorylation, using the energy released by movement of protons across the membrane down their electrochemical gradient, is an example of _____.
A) a reaction with a positive ΔG
B) allosteric regulation
C) an endergonic reaction coupled to an exergonic reaction
D) active transport
C
24) You have a friend who lost 7 kg (about 15 pounds) of fat on a
regimen of strict diet and exercise. How did the fat leave his body?
A) It was converted to heat and then released.
B) It was
released as CO2 and H2O.
C) It was converted to ATP, which weighs
much less than fat.
D) It was converted to urine and eliminated
from the body.
B
25) One function of both alcohol fermentation and lactic acid fermentation is to _____.
A) reduce FAD+ to FADH2 B) reduce NAD+ to NADH
C) oxidize NADH
to NAD+ D) reduce FADH2 to FAD+
C
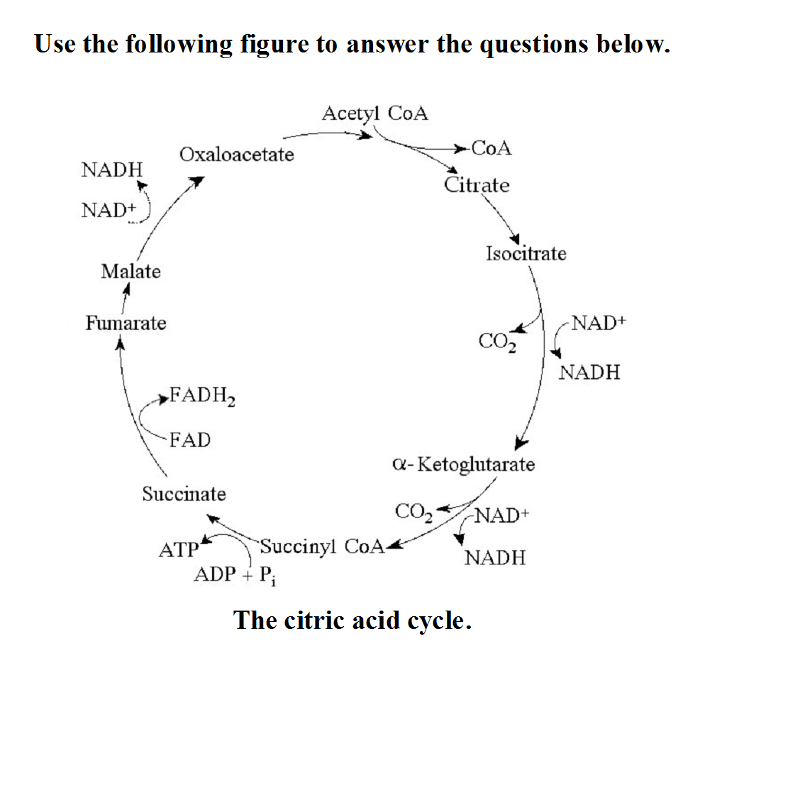
Use the following figure to answer the questions below.
The citric acid cycle.
26) If pyruvate oxidation is blocked,
what will happen to the levels of oxaloacetate and citric acid in the
citric acid cycle shown in the accompanying figure?
A)
Oxaloacetate will accumulate and citric acid will decrease.
B)
Both oxaloacetate and citric acid will accumulate.
C)
Oxaloacetate will decrease and citric acid will accumulate.
D)
Both oxaloacetate and citric acid will decrease.
A
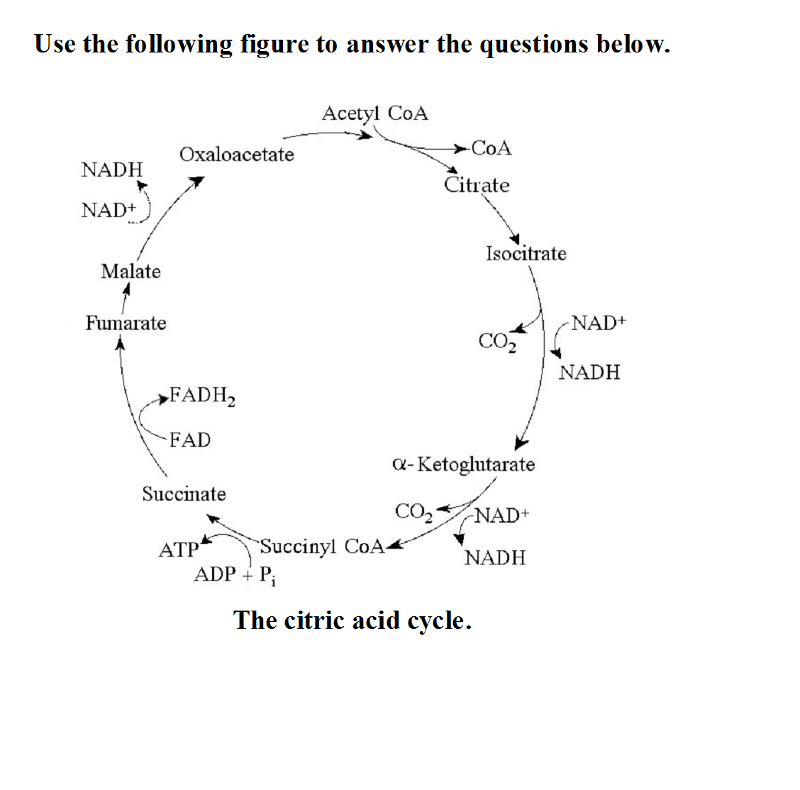
27) Starting with citrate, which of the following combinations of products would result from three acetyl CoA molecules entering the citric acid cycle (see the accompanying figure)?
A) 1 ATP, 2 CO2, 3 NADH, and 1 FADH2 B) 38 ATP, 6 CO2, 3 NADH, and
12 FADH2
C) 3 ATP, 3 CO2, 3 NADH, and 3 FADH2 D) 3 ATP, 6 CO2, 9
NADH, and 3 FADH2
D
28) Chemiosmotic ATP synthesis (oxidative phosphorylation) occurs in
_____.
A) only eukaryotic cells, in the presence of
oxygen
B) all cells, but only in the presence of oxygen
C)
only in mitochondria, using either oxygen or other electron
acceptors
D) all respiring cells, both prokaryotic and
eukaryotic, using either oxygen or other electron acceptors
D
29) What is the oxidizing agent in the following reaction: Pyruvate +
NADH + H+ → Lactate + NAD+
A) pyruvate B) NADH C) NAD+ D) lactate
A
30) Glycolysis is active when cellular energy levels are _____; the regulatory enzyme, phosphofructokinase, is _____ by ATP. A) high; inhibited B) low; activated C) high; activated D) low; inhibited
D
31) Even though plants cells photosynthesize, they still use their
mitochondria for oxidation of pyruvate. This will occur in _____.
A) cells that are storing glucose only
B) photosynthesizing
cells in the light and in other tissues in the dark
C)
photosynthetic cells in the light, while photosynthesis occurs
concurrently
D) all cells all the time
D
32) Some photosynthetic organisms contain chloroplasts that lack
photosystem II, yet are able to survive. The best way to detect the
lack of photosystem II in these organisms would be to _____.
A)
test for liberation of O2 in the light
B) test for CO2 fixation
in the dark
C) do experiments to generate an action
spectrum
D) determine if they have thylakoids in the chloroplasts
A
33) As a research scientist, you measure the amount of ATP and NADPH
consumed by the Calvin cycle in 1 hour. You find that 30,000 molecules
of ATP were consumed, but only 20,000 molecules of NADPH were
consumed. Where did the extra ATP molecules come from?
A) cyclic
electron flow B) photosystem II C) linear electron flow D) photosystem I
A
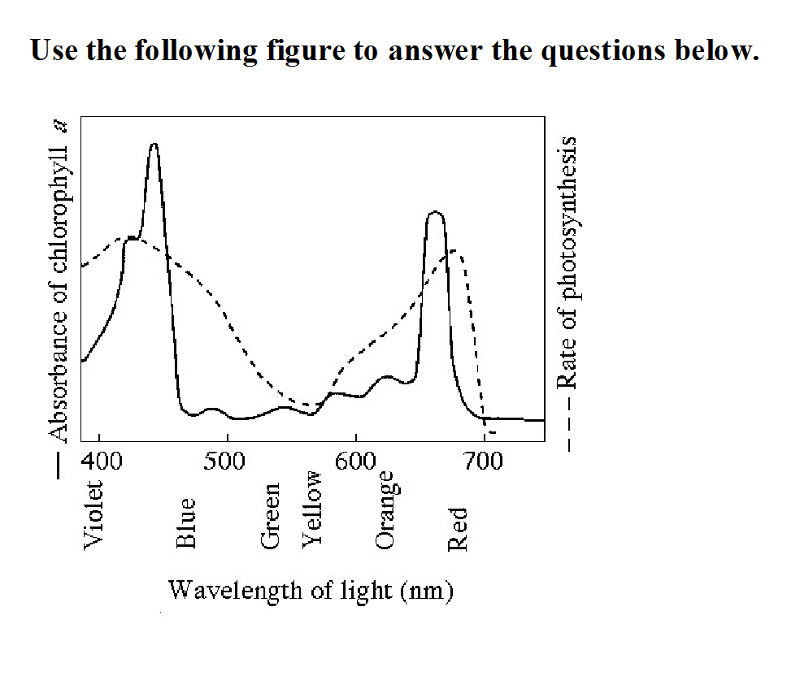
Use the following figure to answer the questions below
34) The figure shows the absorption spectrum for chlorophyll a and
the action spectrum for photosynthesis. Why are they different?
A) Aerobic bacteria take up oxygen, which changes the
measurement of the rate of photosynthesis.
B) Green and yellow
wavelengths inhibit the absorption of red and blue
wavelengths.
C) Other pigments absorb light in addition to
chlorophyll a.
D) Oxygen given off during photosynthesis
interferes with the absorption of light.
C
35) P680+ is said to be the strongest biological oxidizing agent. Given its function, why is this necessary?
A) It obtains electrons from the oxygen atom in a water molecule,
so it must have a stronger attraction for electrons than oxygen
has.
B) It transfers its electrons to reduce NADP+ to
NADPH.
C) It is the molecule that transfers electrons to
plastoquinone (Pq) of the electron transfer system.
D) It is the
receptor for the most excited electron in either photosystem of photosynthesis.
A
36) Which process is most directly driven by light energy?
A)
reduction of NADP+ molecules
B) creation of a pH gradient by
pumping protons across the thylakoid membrane
C) carbon fixation
in the stroma
D) removal of electrons from chlorophyll molecules
D
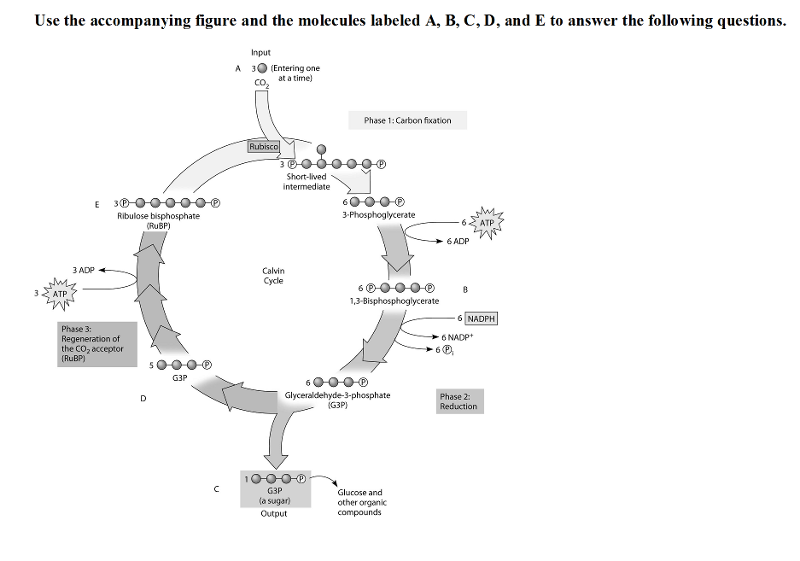
Use the accompanying figure and the molecules labeled A, B, C, D, and E to answer the following questions.
37) Refer to the figure. If the carbon atom of each of the incoming CO2 molecules is labeled with a radioactive isotope of carbon, which organic molecules will be radioactively labeled after one cycle?
A) B and C only B) C, D, and E only C) C only D) B, C, D, and E
D
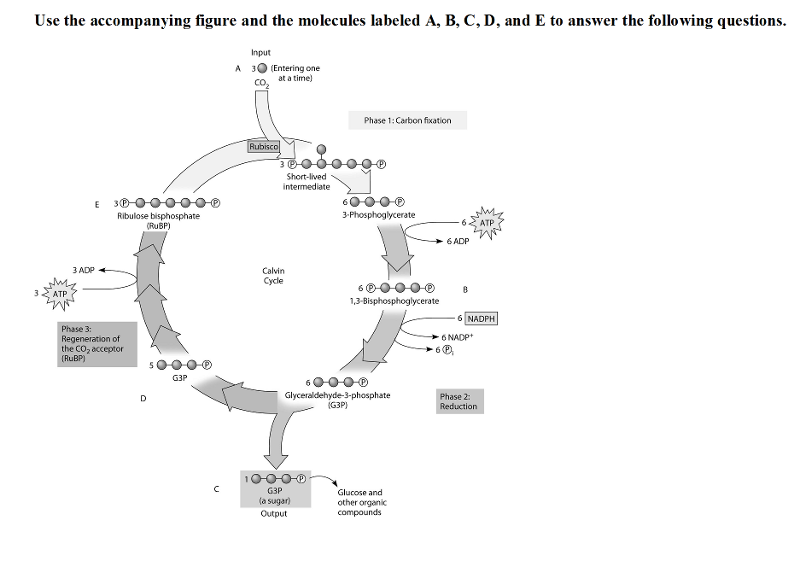
38) Refer to the figure. To identify the molecule that accepts CO2,
Calvin and Benson manipulated the carbon-fixation cycle by either
cutting off CO2 or cutting off light from cultures of photosynthetic
algae. They then measured the concentrations of various metabolites
immediately following the manipulation. How would these experiments
help identify the CO2 acceptor?
A) The CO2 acceptor
concentration would decrease when either the CO2 or light are cut
off.
B) The CO2 acceptor concentration would increase when the
CO2 is cut off, but decrease when the light is cut off.
C) The
CO2 acceptor concentration would decrease when the CO2 is cut off, but
increase when the light is cut off.
D) The CO2 acceptor
concentration would increase when either the CO2 or light are cut off.
B
39) Hormones are chemical substances produced in one organ that are
released into the bloodstream and affect the function of a target
organ. For the target organ to respond to a particular hormone, it
must _____.
A) be from the same cell type as the organ that
produced the hormone
B) have receptors that recognize and bind
the hormone molecule
C) experience an imbalance that disrupts its
normal function
D) modify its plasma membrane to alter the
hormone entering the cytoplasm
B
40) A G-protein receptor with GTP bound to it _____.
A) is in
its active state
B) signals a protein to maintain its shape and
conformation
C) directly affects gene expression
D) will use
cGMP as a second messenger
A
41) Testosterone functions inside a cell by _____.
A)
coordinating a phosphorylation cascade that increases
spermatogenesis
B) acting as a signal receptor that activates
tyrosine kinases
C) acting as a steroid signal receptor that
activates ion channel proteins
D) binding with a receptor protein
that enters the nucleus and activates specific genes
D
42) Which of the following is the best explanation for the inability
of a specific animal cell to reduce the Ca2+ concentration in its
cytosol compared with the extracellular fluid?
A) loss of
transcription factors B) low levels of protein kinase in the
cell
C) insufficient ATP levels in the cytosol D) blockage of the
synaptic signal
C
43) Caffeine is an inhibitor of phosphodiesterase. Therefore, the
cells of a person who has recently consumed coffee would have
increased levels of _____.
A) activated G proteins B) adenylyl
cyclase C) phosphorylated proteins D) cAMP
D
44) Protein kinase is an enzyme that _____.
A) activates or
inactivates other proteins by adding a phosphate group to them
B)
produces second messenger molecules
C) serves as a receptor for
various signal molecules
D) functions as a second messenger molecule
A
45) At puberty, an adolescent female body changes in both structure
and function of several organ systems, primarily under the influence
of changing concentrations of estrogens and other steroid hormones.
How can one hormone, such as estrogen, mediate so many effects?
A) Each cell responds in the same way when steroids bind to the
cell surface.
B) Estrogen is produced in very large concentration
by nearly every tissue of the body.
C) Estrogen is kept away from
the surface of any cells not able to bind it at the surface.
D)
Estrogen binds to specific receptors inside many kinds of cells, each
with different responses.
D
46) Transcription factors _____.
A) regulate the synthesis of
lipids in the cytoplasm B) control gene expression
C) regulate
the synthesis of DNA in response to a signal D) transcribe ATP into cAMP
B
47) An inhibitor of which of the following could be used to block the
release of calcium from the endoplasmic reticulum?
A)
phospholipase C B) adenylyl cyclase C) phosphodiesterase D)
serine/threonine kinases
A
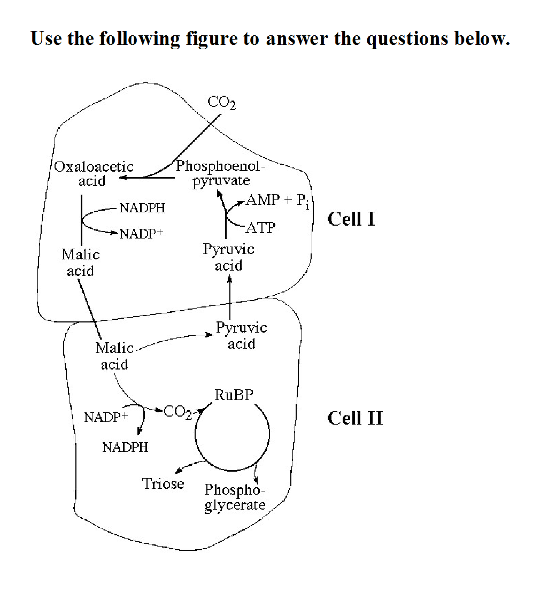
Use the following figure to answer the questions below.
48) Which of the following statements is true concerning the
accompanying figure?
A) It represents a C3 photosynthetic
system.
B) It represents a C4 photosynthetic system.
C) It
represents a CAM photosynthetic system.
D) It represents an
adaptation that maximizes photorespiration.
B
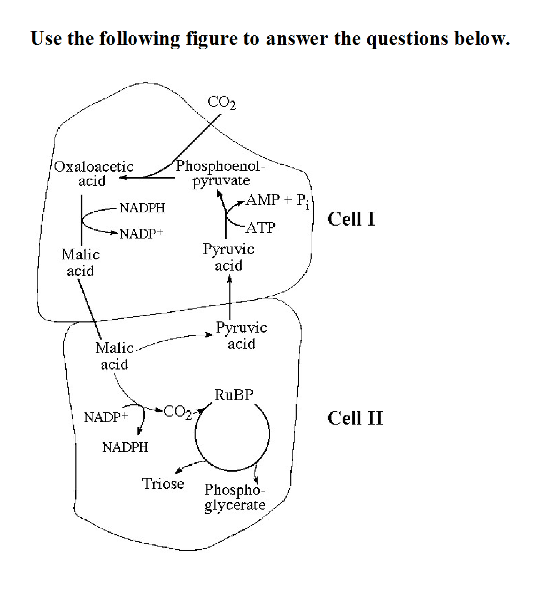
49) Referring to the accompanying figure, oxygen would inhibit the
CO2 fixation reactions in _____.
A) both cell I and cell II B)
cell II only C) cell I only D) neither cell I nor cell II
B
50) Scaffolding proteins are _____.
A) large molecules to which
several relay proteins attach to facilitate cascade effects
B)
microtubular protein arrays that allow lipid-soluble hormones to get
from the cell membrane to the nuclear pores
C) relay proteins
that orient receptors and their ligands in appropriate directions to
facilitate their complexing
D) proteins that can reach into the
nucleus of a cell to affect transcription
A