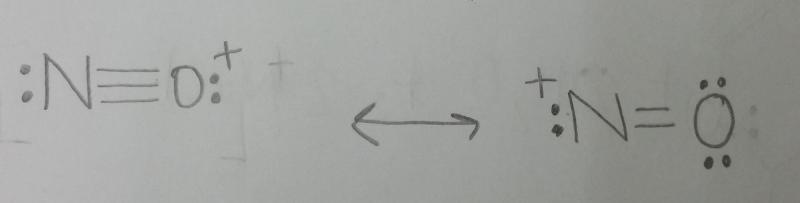
Draw two resonance structures for the nitrosonium ion, NO+.
What is the equation for generating NO+ in a dilute solution of HCl and NaONO?
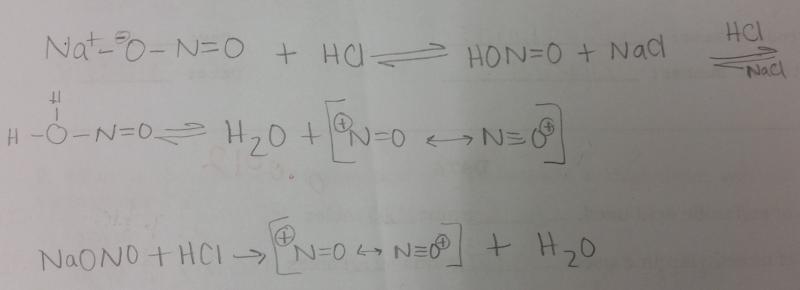
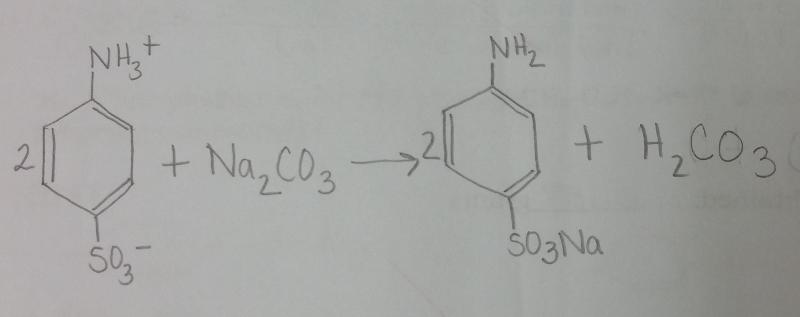
What is the balanced equation for the reaction between sulfanilic acid and sodium carbonate?
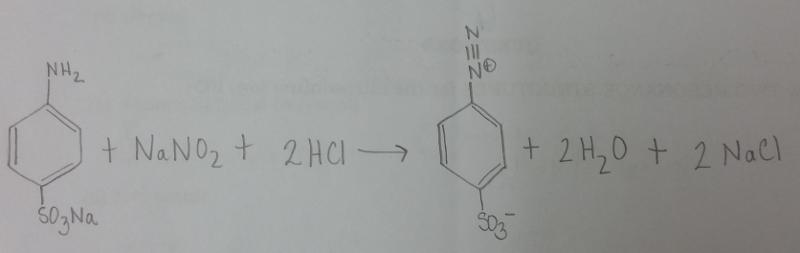
What is the balanced equation for the reaction of sodium sulfanilate, NaONO, and HCl?
What is the balanced equation for the reaction between diazonium salt and N,N,-dimethylaniline?
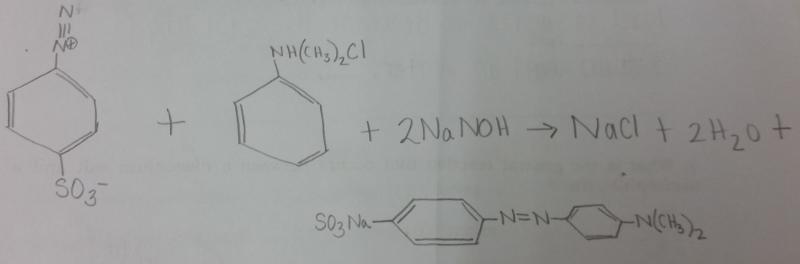
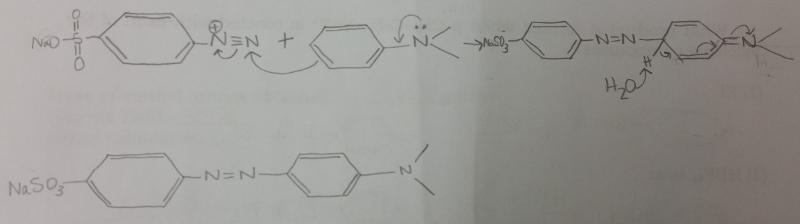
Show by using arrows the movement of electron pairs when the diazonium ion, attacking as an electrophile, attacks N,N,-dimethylaniline. The product of the attack is resonance-stabalized aromatic carbocation.
What is the purpose of adding NaOH to the red crystals of methyl orange?
NaOH is added to ensure that the methyl orange is alkaline. It also neutralizes the HCl so a salt can form.
Why is NaCl added to the reaction mixture before final crystallization?
NaCl is added to decrease the solubility of the sodium salt in water.
What is the general reaction that occurs between diazonium salt and a nucleophile, Nu-?
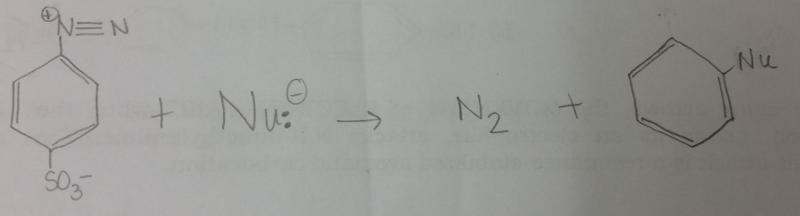
What is the product between diazonium and KI?
What is the product between diazonium and HBF4 and heat?

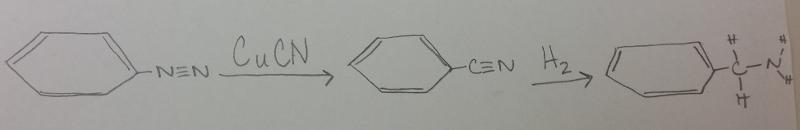
What is the product between diazonium and CuCN, then H2 catalyst?
What is the product between diazonium and phenol, then H2 catalyst?

What is the product between diazonium and o-methylphenol (o-cresol), then H2 catalyst?
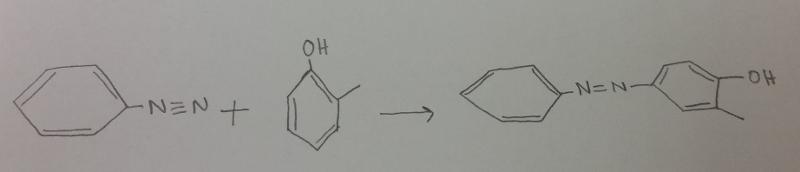
What is the product between diazonium H+, and water?

benzene diazonium
...
diazonium
...