Which term most precisely describes the cellular process of breaking down large molecules into smaller ones?A) catalysisB) metabolism C) anabolism D) dehydration E) catabolism
Answer: E
Which of the following is (are) true for anabolic pathways?
A)
They do not depend on enzymes.
B) They are usually highly
spontaneous chemical reactions.
C) They consume energy to build
up polymers from monomers.
D) They release energy as they degrade
polymers to monomers.
E) They consume energy to decrease the
entropy of the organism and its environment.
Answer: C
Which of the following is a statement of the first law of
thermodynamics?
A) Energy cannot be created or destroyed.
B)
The entropy of the universe is decreasing.
C) The entropy of the
universe is constant.
D) Kinetic energy is stored energy that
results from the specific arrangement of matter.
E) Energy cannot
be transferred or transformed.
Answer: A
For living organisms, which of the following is an important
consequence of the first law of thermodynamics?
A) The energy
content of an organism is constant.
B) The organism ultimately
must obtain all of the necessary energy for life from its
environment.
C) The entropy of an organism decreases with time as
the organism grows in complexity.
D) Organisms grow by converting
energy into organic matter.
E) Life does not obey the first law
of thermodynamics.
Answer: B
Living organisms increase in complexity as they grow, resulting in a
decrease in the entropy of an organism. How does this relate to the
second law of thermodynamics?
A) Living organisms do not obey the
second law of thermodynamics, which states that entropy must increase
with time.
B) Life obeys the second law of thermodynamics because
the decrease in entropy as the organism grows is exactly balanced by
an increase in the entropy of the universe.
C) Living organisms
do not follow the laws of thermodynamics.
D) As a consequence of
growing, organisms cause a greater increase in entropy in their
environment than the decrease in entropy associated with their
growth.
E) Living organisms are able to transform energy into entropy.
Answer: D
Whenever energy is transformed, there is always an increase in
the
A) free energy of the system.
B) free energy of the
universe.
C) entropy of the system.
D) entropy of the
universe.
E) enthalpy of the universe.
Answer: D
Which of the following statements is a logical consequence of the
second law of thermodynamics?
A) If the entropy of a system
increases, there must be a corresponding decrease in the entropy of
the universe.
B) If there is an increase in the energy of a
system, there must be a corresponding decrease in the energy of the
rest of the universe.
C) Every energy transfer requires
activation energy from the environment.
D) Every chemical
reaction must increase the total entropy of the universe.
E)
Energy can be transferred or transformed, but it cannot be created or destroyed.
Answer: D
Which of the following statements is representative of the second law
of thermodynamics?
A) Conversion of energy from one form to
another is always accompanied by some gain of free energy.
B)
Heat represents a form of energy that can be used by most organisms to
do work.
C) Without an input of energy, organisms would tend
toward decreasing entropy.
D) Cells require a constant input of
energy to maintain their high level of organization.
E) Every
energy transformation by a cell decreases the entropy of the universe.
Answer: D
Which of the following types of reactions would decrease the entropy
within a cell?
A) anabolic reactions
B) hydrolysis
C)
respiration
D) digestion
E) catabolic reactions
Answer: A
Biological evolution of life on Earth, from simple prokaryote-like
cells to large, multicellar eukaryotic organisms,
A) has occurred
in accordance with the laws of thermodynamics.
B) has caused an
increase in the entropy of the planet.
C) has been made possible
by expending Earth's energy resources.
D) has occurred in
accordance with the laws of thermodynamics, by expending Earth's
energy resources and causing an increase in the entropy of the
planet.
E) violates the laws of thermodynamics because Earth is a
closed system.
Answer: A
Which of the following is an example of potential rather than kinetic
energy?
A) the muscle contractions of a person mowing
grass
B) water rushing over Niagara Falls
C) light flashes
emitted by a firefly
D) a molecule of glucose
E) the flight
of an insect foraging for food
Answer: D
Which of the following is the smallest closed system?
A) a
cell
B) an organism
C) an ecosystem
D) Earth
E)
the universe
Answer: E
Which of the following is true of metabolism in its entirety in all
organisms?
A) Metabolism depends on a constant supply of energy
from food.
B) Metabolism depends on an organism's adequate
hydration.
C) Metabolism uses all of an organism's
resources.
D) Metabolism consists of all the energy
transformation reactions in an organism.
E) Metabolism manages
the increase of entropy in an organism.
Answer: D
The mathematical expression for the change in free energy of a system
is ΔG =ΔH - TΔS. Which of the following is (are) correct?
A) ΔS
is the change in enthalpy, a measure of randomness.
B) ΔH is the
change in entropy, the energy available to do work.
C) ΔG is the
change in free energy.
D) T is the temperature in degrees Celsius.
Answer: C
A system at chemical equilibrium
A) consumes energy at a steady
rate.
B) releases energy at a steady rate.
C) consumes or
releases energy, depending on whether it is exergonic or
endergonic.
D) has zero kinetic energy.
E) can do no work.
Answer: E
Which of the following is true for all exergonic reactions?
A)
The products have more total energy than the reactants.
B) The
reaction proceeds with a net release of free energy.
C) The
reaction goes only in a forward direction: all reactants will be
converted to products, but no products will be converted to
reactants.
D) A net input of energy from the surroundings is
required for the reactions to proceed.
E) The reactions are rapid.
Answer: B
Chemical equilibrium is relatively rare in living cells. Which of the
following could be an example of a reaction at chemical equilibrium in
a cell?
A) a reaction in which the free energy at equilibrium is
higher than the energy content at any point away from
equilibrium
B) a chemical reaction in which the entropy change in
the reaction is just balanced by an opposite entropy change in the
cell's surroundings
C) an endergonic reaction in an active
metabolic pathway where the energy for that reaction is supplied only
by heat from the environment
D) a chemical reaction in which both
the reactants and products are not being produced or used in any
active metabolic pathway
E) no possibility of having chemical
equilibrium in any living cell
Answer: D
Which of the following shows the correct changes in thermodynamic
properties for a chemical reaction in which amino acids are linked to
form a protein?
A) +ΔH, +ΔS, +ΔG
B) +ΔH, -ΔS, -ΔG
C)
+ΔH, -ΔS, +ΔG
D) -ΔH, -ΔS, +ΔG
E) -ΔH, +ΔS, +ΔG
Answer: C
When glucose monomers are joined together by glycosidic linkages to
form a cellulose polymer, the changes in free energy, total energy,
and entropy are as follows:
A) +ΔG, +ΔH, +ΔS.
B) +ΔG, +ΔH,
-ΔS.
C) +ΔG, -ΔH, -ΔS.
D) -ΔG, +ΔH, +ΔS.
E) -ΔG, -ΔH, -ΔS.
Answer: B
A chemical reaction that has a positive ΔG is correctly described
as
A) endergonic.
B) endothermic.
C) enthalpic.
D)
spontaneous.
E) exothermic.
Answer: A
Which of the following best describes enthalpy (H)?
A) the total
kinetic energy of a system
B) the heat content of a chemical
system
C) the system's entropy
D) the cell's energy
equilibrium
E) the condition of a cell that is not able to react
Answer: B
For the hydrolysis of ATP to ADP + Pi, the free energy change is -7.3
kcal/mol under standard conditions (1 M concentration of both
reactants and products). In the cellular environment, however, the
free energy change is about -13 kcal/mol. What can we conclude about
the free energy change for the formation of ATP from ADP and Pi under
cellular conditions?
A) It is +7.3 kcal/mol.
B) It is less
than +7.3 kcal/mol.
C) It is about +13 kcal/mol.
D) It is
greater than +13 kcal/mol.
E) The information given is
insufficient to deduce the free energy change.
Answer: C
Why is ATP an important molecule in metabolism?
A) Its
hydrolysis provides an input of free energy for exergonic
reactions.
B) It provides energy coupling between exergonic and
endergonic reactions.
C) Its terminal phosphate group contains a
strong covalent bond that, when hydrolyzed, releases free
energy.
D) Its terminal phosphate bond has higher energy than the
other two.
E) It is one of the four building blocks for DNA synthesis.
Answer: B
When 10,000 molecules of ATP are hydrolyzed to ADP and Pi in a test
tube, about twice as much heat is liberated as when a cell hydrolyzes
the same amount of ATP. Which of the following is the best explanation
for this observation?
A) Cells are open systems, but a test tube
is a closed system.
B) Cells are less efficient at heat
production than nonliving systems.
C) The hydrolysis of ATP in a
cell produces different chemical products than does the reaction in a
test tube.
D) The reaction in cells must be catalyzed by enzymes,
but the reaction in a test tube does not need enzymes.
E)
Reactant and product concentrations in the test tube are different
from those in the cell.
Answer: E
Which of the following is most similar in structure to ATP?
A) a
pentose sugar
B) a DNA nucleotide
C) an RNA
nucleotide
D) an amino acid with three phosphate groups
attached
E) a phospholipid
Answer: C
Which of the following statements is true concerning catabolic
pathways?
A) They combine molecules into more energy-rich
molecules.
B) They supply energy, primarily in the form of ATP,
for the cell's work.
C) They are endergonic.
D) They are
spontaneous and do not need enzyme catalysis.
E) They build up
complex molecules such as protein from simpler compounds.
Answer: B
When chemical, transport, or mechanical work is done by an organism,
what happens to the heat generated?
A) It is used to power yet
more cellular work.
B) It is used to store energy as more
ATP.
C) It is used to generate ADP from nucleotide
precursors.
D) It is lost to the environment.
E) It is
transported to specific organs such as the brain.
Answer: D
When ATP releases some energy, it also releases inorganic phosphate.
What purpose does this serve (if any) in the cell?
A) The
phosphate is released as an excretory waste.
B) The phosphate can
only be used to regenerate more ATP.
C) The phosphate can be
added to water and excreted as a liquid.
D) The phosphate may be
incorporated into any molecule that contains phosphate.
E) It
enters the nucleus to affect gene expression.
Answer: D
A number of systems for pumping ions across membranes are powered by
ATP. Such ATP-powered pumps are often called ATPases although they
don't often hydrolyze ATP unless they are simultaneously transporting
ions. Because small increases in calcium ions in the cytosol can
trigger a number of different intracellular reactions, cells keep the
cytosolic calcium concentration quite low under normal conditions,
using ATP-powered calcium pumps. For example, muscle cells transport
calcium from the cytosol into the membranous system called the
sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a
free calcium ion concentration of 10⁻⁷ while the concentration in the
SR is 10⁻², then how is the ATPase acting?
A) ATPase activity
must be powering an inflow of calcium from the outside of the cell
into the SR.
B) ATPase activity must be transferring Pi to the SR
to enable this to occur.
C) ATPase activity must be pumping
calcium from the cytosol to the SR against the concentration
gradient.
D) ATPase activity must be opening a channel for the
calcium ions to diffuse back into the SR along the concentration
gradient.
E) ATPase activity must be routing calcium ions from
the SR to the cytosol, and then to the cell's environment.
Answer: C
What is the difference (if any) between the structure of ATP and the
structure of the precursor of the A nucleotide in RNA?
A) The
sugar molecule is different.
B) The nitrogen-containing base is
different.
C) The number of phosphates is three instead of
one.
D) The number of phosphates is three instead of two.
E)
There is no difference.
Answer: E
Which of the following statements is true about enzyme-catalyzed
reactions?
A) The reaction is faster than the same reaction in
the absence of the enzyme.
B) The free energy change of the
reaction is opposite from the reaction that occurs in the absence of
the enzyme.
C) The reaction always goes in the direction toward
chemical equilibrium.
D) Enzyme-catalyzed reactions require
energy to activate the enzyme.
E) Enzyme-catalyzed reactions
release more free energy than noncatalyzed reactions.
Answer: A
Reactants capable of interacting to form products in a chemical
reaction must first overcome a thermodynamic barrier known as the
reaction's
A) entropy.
B) activation energy.
C)
endothermic level.
D) equilibrium point.
E) free-energy content.
Answer: B
A solution of starch at room temperature does not readily decompose
to form a solution of simple sugars because
A) the starch
solution has less free energy than the sugar solution.
B) the
hydrolysis of starch to sugar is endergonic.
C) the activation
energy barrier for this reaction cannot be surmounted.
D) starch
cannot be hydrolyzed in the presence of so much water.
E) starch
hydrolysis is nonspontaneous.
Answer: C
Which of the following statements regarding enzymes is true?
A)
Enzymes increase the rate of a reaction by making the reaction more
exergonic.
B) Enzymes increase the rate of a reaction by lowering
the activation energy barrier.
C) Enzymes increase the rate of a
reaction by reducing the rate of reverse reactions.
D) Enzymes
change the equilibrium point of the reactions they catalyze.
E)
Enzymes make the rate of a reaction independent of substrate concentrations.
Answer: B
During a laboratory experiment, you discover that an enzyme-catalyzed
reaction has a ∆G of -20 kcal/mol. If you double the amount of enzyme
in the reaction, what will be the ∆G for the new reaction?
A) -40
kcal/mol
B) -20 kcal/mol
C) 0 kcal/mol
D) +20
kcal/mol
E) +40 kcal/mol
Answer: B
The active site of an enzyme is the region that
A) binds
allosteric regulators of the enzyme.
B) is involved in the
catalytic reaction of the enzyme.
C) binds noncompetitive
inhibitors of the enzyme.
D) is inhibited by the presence of a
coenzyme or a cofactor
Answer: B
According to the induced fit hypothesis of enzyme catalysis, which of
the following is correct?
A) The binding of the substrate depends
on the shape of the active site.
B) Some enzymes change their
structure when activators bind to the enzyme.
C) A competitive
inhibitor can outcompete the substrate for the active site.
D)
The binding of the substrate changes the shape of the enzyme's active
site.
E) The active site creates a microenvironment ideal for the reaction.
Answer: D
Mutations that result in single amino acid substitutions in an
enzyme
A) can have no effect on the activity or properties of the
enzyme.
B) will almost always destroy the activity of the
enzyme.
C) will often cause a change in the substrate specificity
of the enzyme.
D) may affect the physicochemical properties of
the enzyme such as its optimal temperature and pH.
E) may, in
rare cases, cause the enzyme to run reactions in reverse
Answer: D
Increasing the substrate concentration in an enzymatic reaction could
overcome which of the following?
A) denaturization of the
enzyme
B) allosteric inhibition
C) competitive
inhibition
D) saturation of the enzyme activity
E)
insufficient cofactors
Answer: C
Which of the following is true of enzymes?
A) Nonprotein
cofactors alter the substrate specificity of enzymes.
B) Enzyme
function is increased if the 3-D structure or conformation of an
enzyme is altered.
C) Enzyme function is independent of physical
and chemical environmental factors such as pH and temperature.
D)
Enzymes increase the rate of chemical reaction by lowering activation
energy barriers.
E) Enzymes increase the rate of chemical
reaction by providing activation energy to the substrate.
Answer: D
Zinc, an essential trace element for most organisms, is present in
the active site of the enzyme carboxypeptidase. The zinc most likely
functions as a(n)
A) competitive inhibitor of the enzyme.
B)
noncompetitive inhibitor of the enzyme.
C) allosteric activator
of the enzyme.
D) cofactor necessary for enzyme activity.
E)
coenzyme derived from a vitamin.
Answer: D
In order to attach a particular amino acid to the tRNA molecule that
will transport it, an enzyme, an aminoacyl-tRNA synthetase, is
required, along with ATP. Initially, the enzyme has an active site for
ATP and another for the amino acid, but it is not able to attach the
tRNA. What must occur in order for the final attachment to
occur?
A) The ATP must first have to attach to the tRNA.
B)
The binding of the first two molecules must cause a 3-D change that
opens another active site on the enzyme.
C) The ATP must be
hydrolyzed to allow the amino acid to bind to the synthetase.
D)
The tRNA molecule must have to alter its shape in order to be able to
fit into the active site with the other two molecules.
E) The 3'
end of the tRNA must have to be cleaved before it can have an attached
amino acid.
Answer: B
Some of the drugs used to treat HIV patients are competitive
inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the
high mutation rate of HIV means that the virus rapidly acquires
mutations with amino acid changes that make them resistant to these
competitive inhibitors. Where in the reverse transcriptase enzyme
would such amino acid changes most likely occur in drug-resistant
viruses?
A) in or near the active site
B) at an allosteric
site
C) at a cofactor binding site
D) in regions of the
protein that determine packaging into the virus capsid
E) such
mutations could occur anywhere with equal probability
Answer: A
Protein kinases are enzymes that transfer the terminal phosphate from
ATP to an amino acid residue on the target protein. Many are located
on the plasma membrane as integral membrane proteins or peripheral
membrane proteins. What purpose may be served by their plasma membrane
localization?
A) ATP is more abundant near the plasma
membrane.
B) They can more readily encounter and phosphorylate
other membrane proteins.
C) Membrane localization lowers the
activation energy of the phosphorylation reaction.
D) They flip
back and forth across the membrane to access target proteins on either
side.
E) They require phospholipids as a cofactor.
Answer: B
When you have a severe fever, what grave consequence may occur if the
fever is not controlled?
A) destruction of your enzymes' primary
structure
B) removal of amine groups from your proteins
C)
change in the tertiary structure of your enzymes
D) removal of
the amino acids in active sites of your enzymes
E) binding of
your enzymes to inappropriate substrates
Answer: C
How does a noncompetitive inhibitor decrease the rate of an enzyme
reaction?
A) by binding at the active site of the enzyme
B)
by changing the shape of the enzyme's active site
C) by changing
the free energy change of the reaction
D) by acting as a coenzyme
for the reaction
E) by decreasing the activation energy of the reaction
Answer: B
The mechanism in which the end product of a metabolic pathway
inhibits an earlier step in the pathway is most precisely described
as
A) metabolic inhibition.
B) feedback inhibition.
C)
allosteric inhibition.
D) noncooperative inhibition.
E)
reversible inhibition.
Answer: B
Which of the following statements describes enzyme
cooperativity?
A) A multienzyme complex contains all the enzymes
of a metabolic pathway.
B) A product of a pathway serves as a
competitive inhibitor of an early enzyme in the pathway.
C) A
substrate molecule bound to an active site of one subunit promotes
substrate binding to the active site of other subunits.
D)
Several substrate molecules can be catalyzed by the same
enzyme.
E) A substrate binds to an active site and inhibits
cooperation between enzymes in a pathway.
Answer: C
Allosteric enzyme regulation is usually associated with
A) lack
of cooperativity.
B) feedback inhibition.
C) activating
activity.
D) an enzyme with more than one subunit.
E) the
need for cofactors.
Answer: D
Which of the following is an example of cooperativity?
A) the
binding of an end product of a metabolic pathway to the first enzyme
that acts in the pathway
B) one enzyme in a metabolic pathway
passing its product to act as a substrate for the next enzyme in the
pathway
C) a molecule binding at one unit of a tetramer, allowing
faster binding at each of the other three
D) the effect of
increasing temperature on the rate of an enzymatic reaction
E)
binding of an ATP molecule along with one of the substrate molecules
in an active site
Answer: C
Protein kinases are enzymes that catalyze phosphorylation of target
proteins at specific sites, whereas protein phosphatases catalyze
removal of phosphate(s) from phosphorylated proteins. Phosphorylation
and dephosphorylation can function as an on-off switch for a protein's
activity, most likely through
A) the change in a protein's charge
leading to a conformational change.
B) the change in a protein's
charge leading to cleavage.
C) a change in the optimal pH at
which a reaction will occur.
D) a change in the optimal
temperature at which a reaction will occur.
E) the excision of
one or more peptides.
Answer: A
Besides turning enzymes on or off, what other means does a cell use
to control enzymatic activity?
A) cessation of cellular protein
synthesis
B) localization of enzymes into specific organelles or
membranes
C) exporting enzymes out of the cell
D) connecting
enzymes into large aggregates
E) hydrophobic interactions
Answer: B
An important group of peripheral membrane proteins are enzymes such
as the phospholipases that cleave the head groups of phospholipids.
What properties must these enzymes exhibit?
A) resistance to
degradation
B) independence from cofactor interaction
C)
water solubility
D) lipid solubility
E) membrane-spanning domains
Answer: C
In experimental tests of enzyme evolution, where a gene encoding an
enzyme is subjected to multiple cycles of random mutagenesis and
selection for altered substrate specificity, the resulting enzyme had
multiple amino acid changes associated with altered substrate
specificity. Where in the enzyme were these amino acid changes
located?
A) only in the active site
B) only in the active
site or near the active site
C) in or near the active site and at
surface sites away from the active site
D) only at surface sites
away from the active site
E) only in the hydrophobic interior of
the folded protein
Answer: C
How might an amino acid change at a site distant from the active site
of the enzyme alter the enzyme's substrate specificity?
A) by
changing the enzyme's stability
B) by changing the enzyme's
location in the cell
C) by changing the shape of the
protein
D) by changing the enzyme's pH optimum
E) an amino
acid change away from the active site cannot alter the enzyme's
substrate specificity
Answer: C
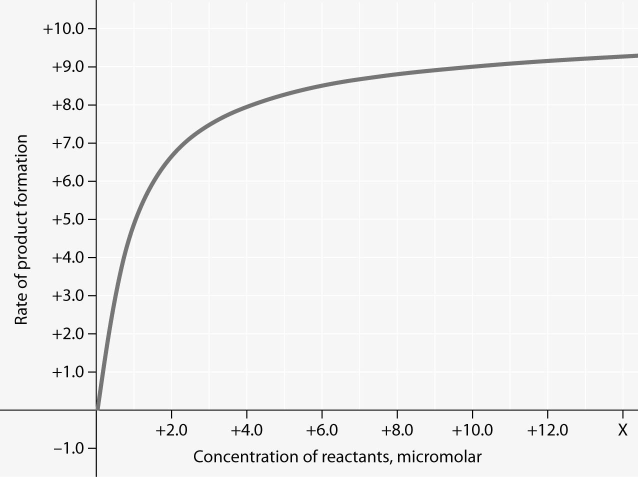
For the enzyme-catalyzed reaction shown in the figure, which of these
treatments will cause the greatest increase in the rate of the
reaction, if the initial reactant concentration is 1.0
micromolar?
A) doubling the activation energy needed
B)
cooling the reaction by 10°C
C) doubling the concentration of the
reactants to 2.0 micromolar
D) doubling the enzyme
concentration
E) increasing the concentration of reactants to
10.0 micromolar, while reducing the concentration of enzyme by 1/2
Answer: D

In the figure, why does the reaction rate plateau at higher reactant
concentrations?
A) Feedback inhibition by product occurs at high
reactant concentrations.
B) Most enzyme molecules are occupied by
substrate at high reactant concentrations.
C) The reaction nears
equilibrium at high reactant concentrations.
D) The activation
energy for the reaction increases with reactant concentration.
E)
The rate of the reverse reaction increases with reactant concentration.
Answer: B
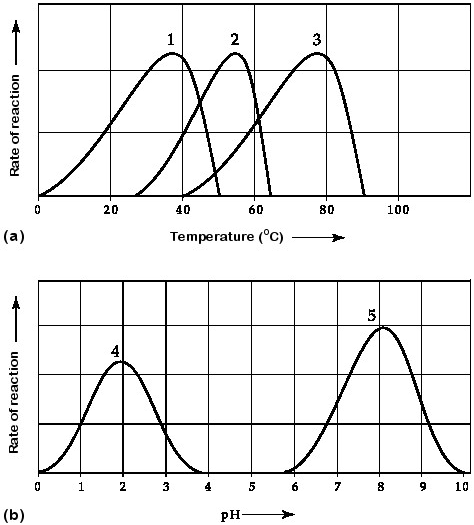
Which curve(s) on the graphs may represent the temperature and pH
profiles of an enzyme taken from a bacterium that lives in a mildly
alkaline hot springs at temperatures of 70°C or higher?
A) curves
1 and 5
B) curves 2 and 4
C) curves 2 and 5
D) curves 3
and 4
E) curves 3 and 5
Answer: E

Which temperature and pH profile curves on the graphs were most
likely generated from analysis of an enzyme from a human stomach where
conditions are strongly acid?
A) curves 1 and 4
B) curves 1
and 5
C) curves 2 and 4
D) curves 2 and 5
E) curves 3
and 4
Answer: A
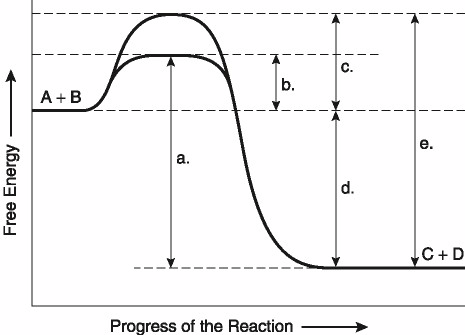
This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following terms best describes the
forward reaction in Figure 8.1?
A) endergonic, ∆G > 0
B)
exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic,
∆G > 0
E) chemical equilibrium, ∆G = 0
Answer: B

This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following represents the ΔG of the
reaction in Figure 8.1?
A) A
B) B
C) C
D)
D
E) E
Answer: D

This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following in Figure 8.1 would be the
same in either an enzyme-catalyzed or a noncatalyzed
reaction?
A) A
B) B
C) C
D) D
E) E
Answer: D

This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following represents the activation
energy needed for the enzyme-catalyzed reverse reaction, C + D → A +
B, in Figure 8.1?
A) A
B) B
C) C
D) D
E) E
Answer: A
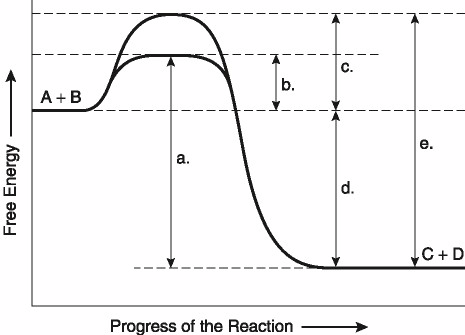
This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following represents the difference
between the free-energy content of the reaction and the free-energy
content of the products in Figure 8.1?
A) A
B) B
C)
C
D) D
E) E
Answer: D
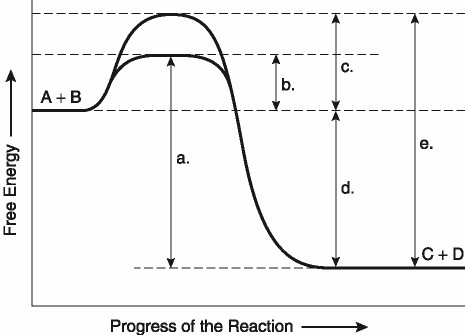
This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following represents the activation
energy required for the enzyme-catalyzed reaction in Figure
8.1?
A) A
B) B
C) C
D) D
E) E
Answer: B

This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following represents the activation
energy required for a noncatalyzed reaction in Figure 8.1?
A)
A
B) B
C) C
D) D
E) E
Answer: C

This question is based on the reaction A + B ↔ C + D shown in the
figure.
Which of the following represents the activation
energy needed for the noncatalyzed reverse reaction, C + D → A + B,
in Figure 8.1?
A) A
B) B
C) C
D) D
E) E
Answer: E

This question is based on the reaction A + B ↔ C + D shown in the
figure.
Assume that the reaction in Figure 8.1 has a ΔG
of -5.6 kcal/mol. Which of the following would be true?
A) The
reaction could be coupled to power an endergonic reaction with a ΔG
of +6.2 kcal/mol.
B) The reaction could be coupled to power an
exergonic reaction with a ΔG of +8.8 kcal/mol.
C) The reaction
would result in a decrease in entropy (S) and an increase in the
total energy content (H) of the system.
D) The reaction would
result in an increase in entropy (S) and a decrease in the total
energy content (H) of the system.
E) The reaction would result
in products (C + D) with a greater free-energy content than in the
initial reactants (A + B).
Answer: D
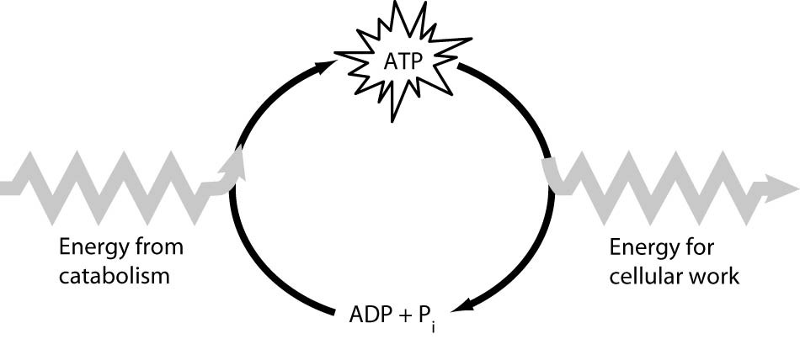
Which of the following is the most correct interpretation of the
figure?
A) Inorganic phosphate is created from organic
phosphate.
B) Energy from catabolism can be used directly for
performing cellular work.
C) ADP + Pi are a set of molecules that
store energy for catabolism.
D) ATP is a molecule that acts as an
intermediary to store energy for cellular work.
E) Pi acts as a
shuttle molecule to move energy from ATP to ADP.
Answer: D

How do cells use the ATP cycle shown in the figure?
A) Cells use
the cycle to recycle ADP and phosphate.
B) Cells use the cycle to
recycle energy released by ATP hydrolysis.
C) Cells use the cycle
to recycle ADP, phosphate, and the energy released by ATP
hydrolysis.
D) Cells use the cycle to generate or consume water
molecules as needed.
E) Cells use the cycle primarily to generate heat.
Answer: A
Succinate dehydrogenase catalyzes the conversion of succinate to
fumarate. The reaction is inhibited by malonic acid, which resembles
succinate but cannot be acted upon by succinate dehydrogenase.
Increasing the ratio of succinate to malonic acid reduces the
inhibitory effect of malonic acid.
Based on this
information, which of the following is correct?
A) Succinate
dehydrogenase is the enzyme, and fumarate is the substrate.
B)
Succinate dehydrogenase is the enzyme, and malonic acid is the
substrate.
C) Succinate is the substrate, and fumarate is the
product.
D) Fumarate is the product, and malonic acid is a
noncompetitive inhibitor.
E) Malonic acid is the product, and
fumarate is a competitive inhibitor.
Answer: C
Succinate dehydrogenase catalyzes the conversion of succinate to
fumarate. The reaction is inhibited by malonic acid, which resembles
succinate but cannot be acted upon by succinate dehydrogenase.
Increasing the ratio of succinate to malonic acid reduces the
inhibitory effect of malonic acid.
What is malonic acid's
role with respect to succinate dehydrogenase?
A) It is a
competitive inhibitor.
B) It blocks the binding of
fumarate.
C) It is a noncompetitive inhibitor.
D) It is
able to bind to succinate.
E) It is an allosteric regulator.
Answer: A
A series of enzymes catalyze the reaction X → Y → Z → A. Product A
binds to the enzyme that converts X to Y at a position remote from its
active site. This binding decreases the activity of the enzyme.
What is substance X?
A) a coenzyme
B) an allosteric
inhibitor
C) a substrate
D) an intermediate
E) the product
Answer: C
A series of enzymes catalyze the reaction X → Y → Z → A. Product A
binds to the enzyme that converts X to Y at a position remote from its
active site. This binding decreases the activity of the enzyme.
With respect to the enzyme that converts X to Y, substance A
functions as
A) a coenzyme.
B) an allosteric
inhibitor.
C) the substrate.
D) an intermediate.
E) a
competitive inhibitor.
Answer: B
Choose the pair of terms that correctly completes this sentence:
Catabolism is to anabolism as ________ is to ________.
A)
exergonic; spontaneous
B) exergonic; endergonic
C) free
energy; entropy
D) work; energy
E) entropy; enthalpy
Answer: B
Most cells cannot harness heat to perform work because
A) heat
is not a form of energy.
B) cells do not have much heat; they are
relatively cool.
C) temperature is usually uniform throughout a
cell.
D) heat can never be used to do work.
E) heat must
remain constant during work.
Answer: C
Which of the following metabolic processes can occur without a net
influx of energy from some other process?
A) ADP + Pi → ATP +
H₂O
B) C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O
C) 6 CO₂ + 6 H₂O →
C₆H₁₂O₆ + 6 O₂
D) amino acids → protein
E) glucose +
fructose → sucrose
Answer: B
If an enzyme in solution is saturated with substrate, the most
effective way to obtain a faster yield of products is to
A) add
more of the enzyme.
B) heat the solution to 90°C.
C) add
more substrate.
D) add an allosteric inhibitor.
E) add a
noncompetitive inhibitor.
Answer: A
Some bacteria are metabolically active in hot springs because
A)
they are able to maintain a lower internal temperature.
B) high
temperatures make catalysis unnecessary.
C) their enzymes have
high optimal temperatures.
D) their enzymes are completely
insensitive to temperature.
E) they use molecules other than
proteins or RNAs as their main catalysts.
Answer: C
If an enzyme is added to a solution where its substrate and product
are in equilibrium, what will occur?
A) Additional product will
be formed.
B) Additional substrate will be formed.
C) The
reaction will change from endergonic to exergonic.
D) The free
energy of the system will change.
E) Nothing; the reaction will
stay at equilibrium.
Answer: E
What is the term for metabolic pathways that release stored energy by
breaking down complex molecules?
A) anabolic pathways
B)
catabolic pathways
C) fermentation pathways
D) thermodynamic
pathways
E) bioenergetic pathways
Answer: B
The molecule that functions as the reducing agent (electron donor) in
a redox or oxidation-reduction reaction
A) gains electrons and
gains potential energy.
B) loses electrons and loses potential
energy.
C) gains electrons and loses potential energy.
D)
loses electrons and gains potential energy.
E) neither gains nor
loses electrons, but gains or loses potential energy.
Answer: B
When electrons move closer to a more electronegative atom, what
happens?
A) The more electronegative atom is reduced, and energy
is released.
B) The more electronegative atom is reduced, and
energy is consumed.
C) The more electronegative atom is oxidized,
and energy is consumed.
D) The more electronegative atom is
oxidized, and energy is released.
E) The more electronegative
atom is reduced, and entropy decreases.
Answer: A
Why does the oxidation of organic compounds by molecular oxygen to
produce CO₂ and water release free energy?
A) The covalent bonds
in organic molecules and molecular oxygen have more kinetic energy
than the covalent bonds in water and carbon dioxide.
B) Electrons
are being moved from atoms that have a lower affinity for electrons
(such as C) to atoms with a higher affinity for electrons (such as
O).
C) The oxidation of organic compounds can be used to make
ATP.
D) The electrons have a higher potential energy when
associated with water and CO₂ than they do in organic
compounds.
E) The covalent bond in O₂ is unstable and easily
broken by electrons from organic molecules.
Answer: B
Which of the following statements describes the results of this
reaction?
C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O + Energy
A) C₆H₁₂O₆
is oxidized and O₂ is reduced.
B) O₂ is oxidized and H₂O is
reduced.
C) CO₂ is reduced and O₂ is oxidized.
D) C₆H₁₂O₆ is
reduced and CO₂ is oxidized.
E) O₂ is reduced and CO₂ is oxidized.
Answer: A
When a glucose molecule loses a hydrogen atom as the result of an
oxidation-reduction reaction, the molecule becomes
A)
hydrolyzed.
B) hydrogenated.
C) oxidized.
D)
reduced.
E) an oxidizing agent.
Answer: C
When a molecule of NAD⁺ (nicotinamide adenine dinucleotide) gains a
hydrogen atom (not a proton), the molecule becomes
A)
dehydrogenated.
B) oxidized.
C) reduced.
D)
redoxed.
E) hydrolyzed.
Answer: C
Which of the following statements describes NAD⁺?
A) NAD⁺ is
reduced to NADH during glycolysis, pyruvate oxidation, and the citric
acid cycle.
B) NAD⁺ has more chemical energy than NADH.
C)
NAD⁺ is oxidized by the action of hydrogenases.
D) NAD⁺ can
donate electrons for use in oxidative phosphorylation.
E) In the
absence of NAD⁺, glycolysis can still function.
Answer: A
Where does glycolysis take place in eukaryotic cells?
A)
mitochondrial matrix
B) mitochondrial outer membrane
C)
mitochondrial inner membrane
D) mitochondrial intermembrane
space
E) cytosol
Answer: E
The ATP made during glycolysis is generated by
A)
substrate-level phosphorylation.
B) electron transport.
C)
photophosphorylation.
D) chemiosmosis.
E) oxidation of NADH
to NAD⁺.
Answer: A
The oxygen consumed during cellular respiration is involved directly
in which process or event?
A) glycolysis
B) accepting
electrons at the end of the electron transport chain
C) the
citric acid cycle
D) the oxidation of pyruvate to acetyl
CoA
E) the phosphorylation of ADP to form ATP
Answer: B
Which process in eukaryotic cells will proceed normally whether
oxygen (O₂) is present or absent?
A) electron transport
B)
glycolysis
C) the citric acid cycle
D) oxidative
phosphorylation
E) chemiosmosis
Answer: B
An electron loses potential energy when it
A) shifts to a less
electronegative atom.
B) shifts to a more electronegative
atom.
C) increases its kinetic energy.
D) increases its
activity as an oxidizing agent.
E) moves further away from the
nucleus of the atom.
Answer: B
Why are carbohydrates and fats considered high energy foods?
A)
They have a lot of oxygen atoms.
B) They have no nitrogen in
their makeup.
C) They can have very long carbon
skeletons.
D) They have a lot of electrons associated with
hydrogen.
E) They are easily reduced.
Answer: D
Substrate-level phosphorylation accounts for approximately what
percentage of the ATP formed by the reactions of glycolysis?
A)
0%
B) 2%
C) 10%
D) 38%
E) 100%
Answer: E
During glycolysis, when each molecule of glucose is catabolized to
two molecules of pyruvate, most of the potential energy contained in
glucose is
A) transferred to ADP, forming ATP.
B)
transferred directly to ATP.
C) retained in the two
pyruvates.
D) stored in the NADH produced.
E) used to
phosphorylate fructose to form fructose 6-phosphate.
Answer: C
In addition to ATP, what are the end products of glycolysis?
A)
CO₂ and H₂O
B) CO₂ and pyruvate
C) NADH and pyruvate
D)
CO₂ and NADH
E) H₂O, FADH₂, and citrate
Answer: C
The free energy for the oxidation of glucose to CO₂ and water is -686
kcal/mol and the free energy for the reduction of NAD⁺ to NADH is +53
kcal/mol. Why are only two molecules of NADH formed during glycolysis
when it appears that as many as a dozen could be formed?
A) Most
of the free energy available from the oxidation of glucose is used in
the production of ATP in glycolysis.
B) Glycolysis is a very
inefficient reaction, with much of the energy of glucose released as
heat.
C) Most of the free energy available from the oxidation of
glucose remains in pyruvate, one of the products of
glycolysis.
D) There is no CO₂ or water produced as products of
glycolysis.
E) Glycolysis consists of many enzymatic reactions,
each of which extracts some energy from the glucose molecule.
Answer: C
Starting with one molecule of glucose, the energy-containing products
of glycolysis are
A) 2 NAD⁺, 2 pyruvate, and 2 ATP.
B) 2
NADH, 2 pyruvate, and 2 ATP.
C) 2 FADH₂, 2 pyruvate, and 4
ATP.
D) 6 CO₂, 2 ATP, and 2 pyruvate.
E) 6 CO₂, 30 ATP, and
2 pyruvate.
Answer: B
In glycolysis, for each molecule of glucose oxidized to
pyruvate
A) two molecules of ATP are used and two molecules of
ATP are produced.
B) two molecules of ATP are used and four
molecules of ATP are produced.
C) four molecules of ATP are used
and two molecules of ATP are produced.
D) two molecules of ATP
are used and six molecules of ATP are produced.
E) six molecules
of ATP are used and six molecules of ATP are produced.
Answer: B
A molecule that is phosphorylated
A) has been reduced as a
result of a redox reaction involving the loss of an inorganic
phosphate.
B) has a decreased chemical reactivity; it is less
likely to provide energy for cellular work.
C) has been oxidized
as a result of a redox reaction involving the gain of an inorganic
phosphate.
D) has an increased chemical potential energy; it is
primed to do cellular work.
E) has less energy than before its
phosphorylation and therefore less energy for cellular work.
Answer: D
Which kind of metabolic poison would most directly interfere with
glycolysis?
A) an agent that reacts with oxygen and depletes its
concentration in the cell
B) an agent that binds to pyruvate and
inactivates it
C) an agent that closely mimics the structure of
glucose but is not metabolized
D) an agent that reacts with NADH
and oxidizes it to NAD⁺
E) an agent that blocks the passage of
electrons along the electron transport chain
Answer: C
Why is glycolysis described as having an investment phase and a
payoff phase?
A) It both splits molecules and assembles
molecules.
B) It attaches and detaches phosphate groups.
C)
It uses glucose and generates pyruvate.
D) It shifts molecules
from cytosol to mitochondrion.
E) It uses stored ATP and then
forms a net increase in ATP.
Answer: E
The transport of pyruvate into mitochondria depends on the
proton-motive force across the inner mitochondrial membrane. How does
pyruvate enter the mitochondrion?
A) active transport
B)
diffusion
C) facilitated diffusion
D) through a
channel
E) through a pore
Answer: A
Which of the following intermediary metabolites enters the citric
acid cycle and is formed, in part, by the removal of a carbon (CO₂)
from one molecule of pyruvate?
A) lactate
B)
glyceraldehydes-3-phosphate
C) oxaloacetate
D) acetyl
CoA
E) citrate
Answer: D
During cellular respiration, acetyl CoA accumulates in which
location?
A) cytosol
B) mitochondrial outer membrane
C)
mitochondrial inner membrane
D) mitochondrial intermembrane
space
E) mitochondrial matrix
Answer: E
How many carbon atoms are fed into the citric acid cycle as a result
of the oxidation of one molecule of pyruvate?
A) two
B)
four
C) six
D) eight
E) ten
Answer: A
Carbon dioxide (CO₂) is released during which of the following stages
of cellular respiration?
A) glycolysis and the oxidation of
pyruvate to acetyl CoA
B) oxidation of pyruvate to acetyl CoA and
the citric acid cycle
C) the citric acid cycle and oxidative
phosphorylation
D) oxidative phosphorylation and
fermentation
E) fermentation and glycolysis
Answer: B
A young animal has never had much energy. He is brought to a
veterinarian for help and is sent to the animal hospital for some
tests. There they discover his mitochondria can use only fatty acids
and amino acids for respiration, and his cells produce more lactate
than normal. Of the following, which is the best explanation of his
condition?
A) His mitochondria lack the transport protein that
moves pyruvate across the outer mitochondrial membrane.
B) His
cells cannot move NADH from glycolysis into the mitochondria.
C)
His cells contain something that inhibits oxygen use in his
mitochondria.
D) His cells lack the enzyme in glycolysis that
forms pyruvate.
E) His cells have a defective electron transport
chain, so glucose goes to lactate instead of to acetyl CoA.
Answer: A
During aerobic respiration, electrons travel downhill in which
sequence?
A) food → citric acid cycle → ATP → NAD⁺
B) food →
NADH → electron transport chain → oxygen
C) glucose → pyruvate →
ATP → oxygen
D) glucose → ATP → electron transport chain →
NADH
E) food → glycolysis → citric acid cycle → NADH → ATP
Answer: B
What fraction of the carbon dioxide exhaled by animals is generated
by the reactions of the citric acid cycle, if glucose is the sole
energy source?
A) 1/6
B) 1/3
C) 1/2
D) 2/3
E) 100/100
Answer: D
Where are the proteins of the electron transport chain
located?
A) cytosol
B) mitochondrial outer membrane
C)
mitochondrial inner membrane
D) mitochondrial intermembrane
space
E) mitochondrial matrix
Answer: C
In cellular respiration, the energy for most ATP synthesis is
supplied by
A) high energy phosphate bonds in organic
molecules.
B) a proton gradient across a membrane.
C)
converting oxygen to ATP.
D) transferring electrons from organic
molecules to pyruvate.
E) generating carbon dioxide and oxygen in
the electron transport chain.
Answer: B
During aerobic respiration, which of the following directly donates
electrons to the electron transport chain at the lowest energy
level?
A) NAD+
B) NADH
C) ATP
D) ADP + Pi
E) FADH2
Answer: E
The primary role of oxygen in cellular respiration is to
A)
yield energy in the form of ATP as it is passed down the respiratory
chain.
B) act as an acceptor for electrons and hydrogen, forming
water.
C) combine with carbon, forming CO₂.
D) combine with
lactate, forming pyruvate.
E) catalyze the reactions of glycolysis.
Answer: B
Inside an active mitochondrion, most electrons follow which
pathway?
A) glycolysis → NADH → oxidative phosphorylation → ATP →
oxygen
B) citric acid cycle → FADH₂ → electron transport chain →
ATP
C) electron transport chain → citric acid cycle → ATP →
oxygen
D) pyruvate → citric acid cycle → ATP → NADH →
oxygen
E) citric acid cycle → NADH → electron transport chain → oxygen
Answer: E
During aerobic respiration, H₂O is formed. Where does the oxygen atom
for the formation of the water come from?
A) carbon dioxide
(CO₂)
B) glucose (C₆H₁₂O₆)
C) molecular oxygen (O₂)
D)
pyruvate (C₃H₃O₃-)
E) lactate (C₃H₅O₃-)
Answer: C
In chemiosmotic phosphorylation, what is the most direct source of
energy that is used to convert ADP + Pi to ATP?
A) energy
released as electrons flow through the electron transport
system
B) energy released from substrate-level
phosphorylation
C) energy released from movement of protons
through ATP synthase, against the electrochemical gradient
D)
energy released from movement of protons through ATP synthase, down
the electrochemical gradient
E) No external source of energy is
required because the reaction is exergonic.
Answer: D
Energy released by the electron transport chain is used to pump H⁺
into which location in eukaryotic cells?
A) cytosol
B)
mitochondrial outer membrane
C) mitochondrial inner
membrane
D) mitochondrial intermembrane space
E)
mitochondrial matrix
Answer: D
The direct energy source that drives ATP synthesis during respiratory
oxidative phosphorylation in eukaryotic cells is
A) oxidation of
glucose to CO₂ and water.
B) the thermodynamically favorable flow
of electrons from NADH to the mitochondrial electron transport
carriers.
C) the final transfer of electrons to oxygen.
D)
the proton-motive force across the inner mitochondrial
membrane.
E) the thermodynamically favorable transfer of
phosphate from glycolysis and the citric acid cycle intermediate
molecules of ADP.
Answer: D
When hydrogen ions are pumped from the mitochondrial matrix across
the inner membrane and into the intermembrane space, the result is
the
A) formation of ATP.
B) reduction of NAD⁺.
C)
restoration of the Na⁺/K⁺ balance across the membrane.
D)
creation of a proton-motive force.
E) lowering of pH in the
mitochondrial matrix.
Answer: D
Where is ATP synthase located in the mitochondrion?
A)
cytosol
B) electron transport chain
C) outer
membrane
D) inner membrane
E) mitochondrial matrix
Answer: D
It is possible to prepare vesicles from portions of the inner
mitochondrial membrane. Which one of the following processes could
still be carried on by this isolated inner membrane?
A) the
citric acid cycle
B) oxidative phosphorylation
C) glycolysis
and fermentation
D) reduction of NAD⁺
E) both the citric
acid cycle and oxidative phosphorylation
Answer: B
How many oxygen molecules (O₂) are required each time a molecule of
glucose (C₆H₁₂O₆) is completely oxidized to carbon dioxide and water
via aerobic respiration,?
A) 1
B) 3
C) 6
D)
12
E) 30
Answer: C
Which of the following produces the most ATP when glucose (C₆H₁₂O₆)
is completely oxidized to carbon dioxide (CO₂) and water?
A)
glycolysis
B) fermentation
C) oxidation of pyruvate to
acetyl CoA
D) citric acid cycle
E) oxidative phosphorylation (chemiosmosis)
Answer: E
Approximately how many molecules of ATP are produced from the
complete oxidation of two molecules of glucose (C₆H₁₂O₆) in aerobic
cellular respiration?
A) 2
B) 4
C) 15
D)
30-32
E) 60-64
Answer: E
The synthesis of ATP by oxidative phosphorylation, using the energy
released by movement of protons across the membrane down their
electrochemical gradient, is an example of
A) active
transport.
B) an endergonic reaction coupled to an exergonic
reaction.
C) a reaction with a positive ΔG .
D)
osmosis.
E) allosteric regulation.
Answer: B
Chemiosmotic ATP synthesis (oxidative phosphorylation) occurs
in
A) all cells, but only in the presence of oxygen.
B) only
eukaryotic cells, in the presence of oxygen.
C) only in
mitochondria, using either oxygen or other electron acceptors.
D)
all respiring cells, both prokaryotic and eukaryotic, using either
oxygen or other electron acceptors.
E) all cells, in the absence
of respiration.
Answer: D
If a cell is able to synthesize 30 ATP molecules for each molecule of
glucose completely oxidized by carbon dioxide and water, how many ATP
molecules can the cell synthesize for each molecule of pyruvate
oxidized to carbon dioxide and water?
A) 0
B) 1
C)
12
D) 14
E) 15
Answer: C
What is proton-motive force?
A) the force required to remove an
electron from hydrogen
B) the force exerted on a proton by a
transmembrane proton concentration gradient
C) the force that
moves hydrogen into the intermembrane space
D) the force that
moves hydrogen into the mitochondrion
E) the force that moves
hydrogen to NAD⁺
Answer: B
In liver cells, the inner mitochondrial membranes are about five
times the area of the outer mitochondrial membranes. What purpose must
this serve?
A) It allows for an increased rate of
glycolysis.
B) It allows for an increased rate of the citric acid
cycle.
C) It increases the surface for oxidative
phosphorylation.
D) It increases the surface for substrate-level
phosphorylation.
E) It allows the liver cell to have fewer mitochondria.
Answer: C
Brown fat cells produce a protein called thermogenin in their
mitochondrial inner membrane. Thermogenin is a channel for facilitated
transport of protons across the membrane. What will occur in the brown
fat cells when they produce thermogenin?
A) ATP synthesis and
heat generation will both increase.
B) ATP synthesis will
increase, and heat generation will decrease.
C) ATP synthesis
will decrease, and heat generation will increase.
D) ATP
synthesis and heat generation will both decrease.
E) ATP
synthesis and heat generation will stay the same.
Answer: C
In a mitochondrion, if the matrix ATP concentration is high, and the
intermembrane space proton concentration is too low to generate
sufficient proton-motive force, then
A) ATP synthase will
increase the rate of ATP synthesis.
B) ATP synthase will stop
working.
C) ATP synthase will hydrolyze ATP and pump protons into
the intermembrane space.
D) ATP synthase will hydrolyze ATP and
pump protons into the matrix.
Answer: C
Which catabolic processes may have been used by cells on ancient
Earth before free oxygen became available?
A) glycolysis and
fermentation only
B) glycolysis and the citric acid cycle
only
C) glycolysis, pyruvate oxidation, and the citric acid
cycle
D) oxidative phosphorylation only
E) glycolysis,
pyruvate oxidation, the citric acid cycle, and oxidative
phosphorylation, using an electron acceptor other than oxygen
Answer: E
Which of the following normally occurs regardless of whether or not
oxygen (O₂) is present?
A) glycolysis
B)
fermentation
C) oxidation of pyruvate to acetyl CoA
D)
citric acid cycle
E) oxidative phosphorylation (chemiosmosis)
Answer: A
Which of the following occurs in the cytosol of a eukaryotic
cell?
A) glycolysis and fermentation
B) fermentation and
chemiosmosis
C) oxidation of pyruvate to acetyl CoA
D)
citric acid cycle
E) oxidative phosphorylation
Answer: A
Which metabolic pathway is common to both cellular respiration and
fermentation?
A) the oxidation of pyruvate to acetyl CoA
B)
the citric acid cycle
C) oxidative phosphorylation
D)
glycolysis
E) chemiosmosis
Answer: D
The ATP made during fermentation is generated by which of the
following?
A) the electron transport chain
B)
substrate-level phosphorylation
C) chemiosmosis
D) oxidative
phosphorylation
E) aerobic respiration
Answer: B
In the absence of oxygen, yeast cells can obtain energy by
fermentation, resulting in the production of
A) ATP, CO₂, and
ethanol (ethyl alcohol).
B) ATP, CO₂, and lactate.
C) ATP,
NADH, and pyruvate.
D) ATP, pyruvate, and oxygen.
E) ATP,
pyruvate, and acetyl CoA.
Answer: A
In alcohol fermentation, NAD⁺ is regenerated from NADH by
A)
reduction of acetaldehyde to ethanol (ethyl alcohol).
B)
oxidation of pyruvate to acetyl CoA.
C) reduction of pyruvate to
form lactate.
D) oxidation of ethanol to acetyl CoA.
E)
reduction of ethanol to pyruvate.
Answer: A
One function of both alcohol fermentation and lactic acid
fermentation is to
A) reduce NAD⁺ to NADH.
B) reduce FAD⁺ to
FADH₂.
C) oxidize NADH to NAD⁺.
D) reduce FADH₂ to
FAD⁺.
E) do none of the above.
Answer: C
An organism is discovered that thrives both in the presence and
absence of oxygen in the air. Curiously, the consumption of sugar
increases as oxygen is removed from the organism's environment, even
though the organism does not gain much weight. This organism
A)
must use a molecule other than oxygen to accept electrons from the
electron transport chain.
B) is a normal eukaryotic
organism.
C) is photosynthetic.
D) is an anaerobic
organism.
E) is a facultative anaerobe.
Answer: E
Which statement best supports the hypothesis that glycolysis is an
ancient metabolic pathway that originated before the last universal
common ancestor of life on Earth?
A) Glycolysis is widespread and
is found in the domains Bacteria, Archaea, and Eukarya.
B)
Glycolysis neither uses nor needs O₂.
C) Glycolysis is found in
all eukaryotic cells.
D) The enzymes of glycolysis are found in
the cytosol rather than in a membrane-enclosed organelle.
E)
Ancient prokaryotic cells, the most primitive of cells, made extensive
use of glycolysis long before oxygen was present in Earth's atmosphere.
Answer: A
Why is glycolysis considered to be one of the first metabolic
pathways to have evolved?
A) It produces much less ATP than does
oxidative phosphorylation.
B) It does not involve organelles or
specialized structures, does not require oxygen, and is present in
most organisms.
C) It is found in prokaryotic cells but not in
eukaryotic cells.
D) It relies on chemiosmosis, which is a
metabolic mechanism present only in the first cells' prokaryotic
cells.
E) It requires the presence of membrane-enclosed cell
organelles found only in eukaryotic cells.
Answer: B
When an individual is exercising heavily and when the muscle becomes
oxygen-deprived, muscle cells convert pyruvate to lactate. What
happens to the lactate in skeletal muscle cells?
A) It is
converted to NAD⁺.
B) It produces CO₂ and water.
C) It is
taken to the liver and converted back to pyruvate.
D) It reduces
FADH₂ to FAD⁺.
E) It is converted to alcohol
Answer: C
When skeletal muscle cells are oxygen-deprived, the heart still
pumps. What must the heart muscle cells be able to do?
A) derive
sufficient energy from fermentation
B) continue aerobic
metabolism when skeletal muscle cannot
C) transform lactate to
pyruvate again
D) remove lactate from the blood
E) remove
oxygen from lactate
Answer: B
When skeletal muscle cells undergo anaerobic respiration, they become
fatigued and painful. This is now known to be caused by
A)
buildup of pyruvate.
B) buildup of lactate.
C) increase in
sodium ions.
D) increase in potassium ions.
E) increase in ethanol.
Answer: B
A mutation in yeast makes it unable to convert pyruvate to ethanol.
How will this mutation affect these yeast cells?
A) The mutant
yeast will be unable to grow anaerobically.
B) The mutant yeast
will grow anaerobically only when given glucose.
C) The mutant
yeast will be unable to metabolize glucose.
D) The mutant yeast
will die because they cannot regenerate NAD⁺ from NAD.
E) The
mutant yeast will metabolize only fatty acids.
Answer: A
You have a friend who lost 7 kg (about 15 pounds) of fat on a regimen
of strict diet and exercise. How did the fat leave her body?
A)
It was released as CO₂ and H₂O.
B) It was converted to heat and
then released.
C) It was converted to ATP, which weighs much less
than fat.
D) It was broken down to amino acids and eliminated
from the body.
E) It was converted to urine and eliminated from
the body.
Answer: A
Phosphofructokinase is an important control enzyme in the regulation
of cellular respiration. Which of the following statements correctly
describes phosphofructokinase activity?
A) It is inhibited by
AMP.
B) It is activated by ATP.
C) It is activated by
citrate, an intermediate of the citric acid cycle.
D) It
catalyzes the conversion of fructose 1,6-bisphosphate to fructose
6-phosphate, an early step of glycolysis.
E) It is an allosteric enzyme.
Answer: E
Phosphofructokinase is an allosteric enzyme that catalyzes the
conversion of fructose 6-phosphate to fructose 1,6-bisphosphate, an
early step of glycolysis. In the presence of oxygen, an increase in
the amount of ATP in a cell would be expected to
A) inhibit the
enzyme and thus slow the rates of glycolysis and the citric acid
cycle.
B) activate the enzyme and thus slow the rates of
glycolysis and the citric acid cycle.
C) inhibit the enzyme and
thus increase the rates of glycolysis and the citric acid
cycle.
D) activate the enzyme and increase the rates of
glycolysis and the citric acid cycle.
E) inhibit the enzyme and
thus increase the rate of glycolysis and the concentration of citrate.
Answer: A
Even though plants carry on photosynthesis, plant cells still use
their mitochondria for oxidation of pyruvate. When and where will this
occur?
A) in photosynthetic cells in the light, while
photosynthesis occurs concurrently
B) in nonphotosynthesizing
cells only
C) in cells that are storing glucose only
D) in
all cells all the time
E) in photosynthesizing cells in the light
and in other tissues in the dark
Answer: D
In vertebrate animals, brown fat tissue's color is due to abundant
blood vessels and capillaries. White fat tissue, on the other hand, is
specialized for fat storage and contains relatively few blood vessels
or capillaries. Brown fat cells have a specialized protein that
dissipates the proton-motive force across the mitochondrial membranes.
Which of the following might be the function of the brown fat
tissue?
A) to increase the rate of oxidative phosphorylation from
its few mitochondria
B) to allow the animals to regulate their
metabolic rate when it is especially hot
C) to increase the
production of ATP
D) to allow other membranes of the cell to
perform mitochondrial functions
E) to regulate temperature by
converting most of the energy from NADH oxidation to heat
Answer: E
What is the purpose of beta oxidation in respiration?
A)
oxidation of glucose
B) oxidation of pyruvate
C) feedback
regulation
D) control of ATP accumulation
E) breakdown of
fatty acids
Answer: E
Where do the catabolic products of fatty acid breakdown enter into
the citric acid cycle?
A) pyruvate
B) malate or
fumarate
C) acetyl CoA
D) α-ketoglutarate
E) succinyl CoA
Answer: C
What carbon sources can yeast cells metabolize to make ATP from ADP
under anaerobic conditions?
A) glucose
B) ethanol
C)
pyruvate
D) lactic acid
E) either ethanol or lactic acid
Answer: A
High levels of citric acid inhibit the enzyme phosphofructokinase, a
key enzyme in glycolysis. Citric acid binds to the enzyme at a
different location from the active site. This is an example of
A)
competitive inhibition.
B) allosteric regulation.
C) the
specificity of enzymes for their substrates.
D) an enzyme
requiring a cofactor.
E) positive feedback regulation.
Answer: B
During intense exercise, as skeletal muscle cells go into
anaerobiosis, the human body will increase its catabolism of
A)
fats only.
B) carbohydrates only.
C) proteins only.
D)
fats, carbohydrates, and proteins.
E) fats and proteins only.
Answer: B
Yeast cells that have defective mitochondria incapable of respiration
will be able to grow by catabolizing which of the following carbon
sources for energy?
A) glucose
B) proteins
C) fatty
acids
D) glucose, proteins, and fatty acids
E) Such yeast
cells will not be capable of catabolizing any food molecules, and will
therefore die.
Answer: A
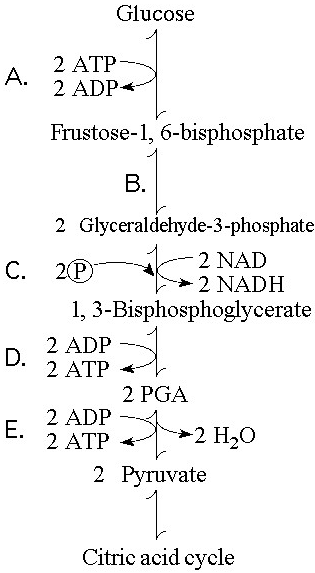
Which step in Figure 9.1 shows a split of one molecule into two
smaller molecules?
A) A
B) B
C) C
D) D
E) E
Answer: B
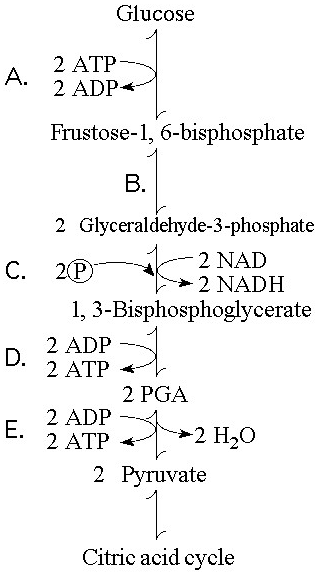
In which step in Figure 9.1 is an inorganic phosphate added to the
reactant?
A) A
B) B
C) C
D) D
E) E
Answer: C

Which step in Figure 9.1 is a redox reaction?
A) A
B)
B
C) C
D) D
E) E
Answer: C
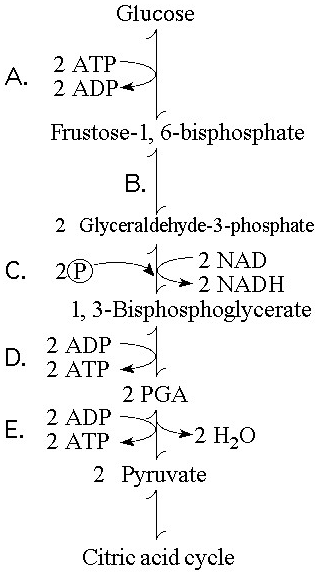
Which portion of the pathway in Figure 9.1 involves an endergonic
reaction?
A) A
B) B
C) C
D) D
E) E
Answer: A
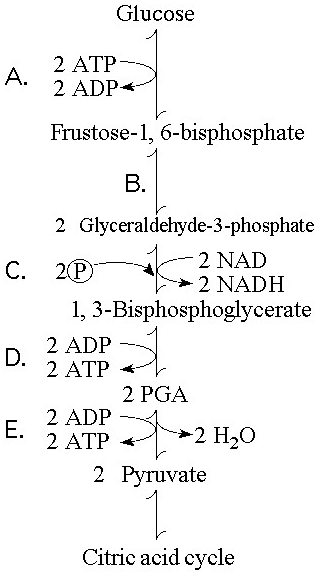
Which portion of the pathway in Figure 9.1 contains a phosphorylation
reaction in which ATP is the phosphate source?
A) A
B)
B
C) C
D) D
E) E
Answer: A
Starting with one molecule of isocitrate and ending with fumarate,
how many ATP molecules can be made through substrate-level
phosphorylation (see Figure 9.2)?
A) 1
B) 2
C)
11
D) 12
E) 24
Answer: A
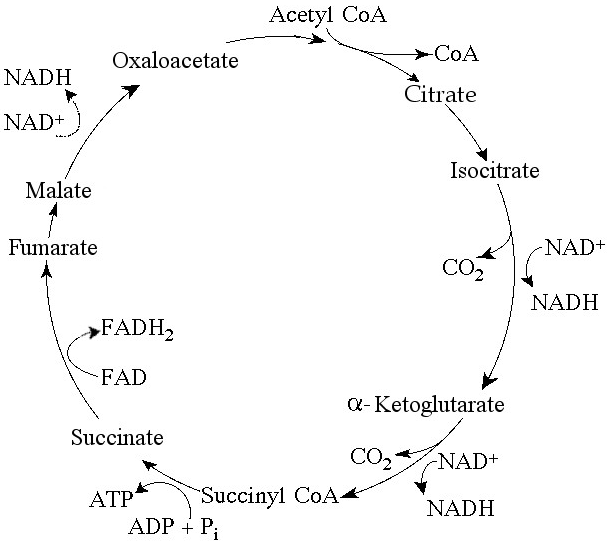
Carbon skeletons for amino acid biosynthesis are supplied by
intermediates of the citric acid cycle. Which intermediate would
supply the carbon skeleton for synthesis of a five-carbon amino acid
(see Figure 9.2)?
A) succinate
B) malate
C)
citrate
D) α-ketoglutarate
E) isocitrate
Answer: D
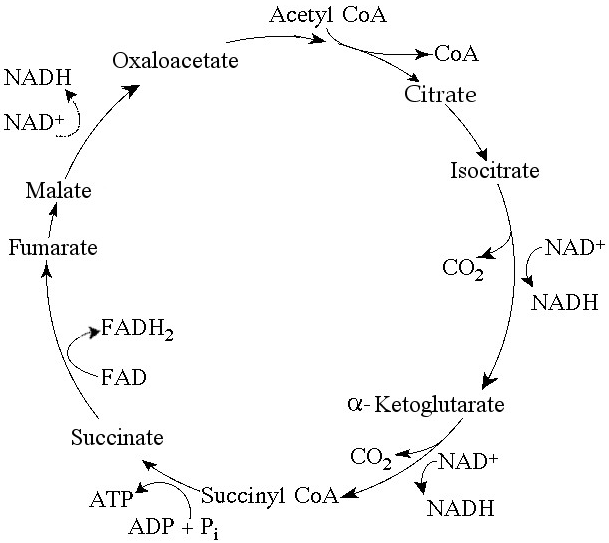
For each mole of glucose (C₆H₁₂O₆) oxidized by cellular respiration,
how many moles of CO₂ are released in the citric acid cycle (see
Figure 9.2)?
A) 2
B) 4
C) 6
D) 12
E) 3
Answer: B
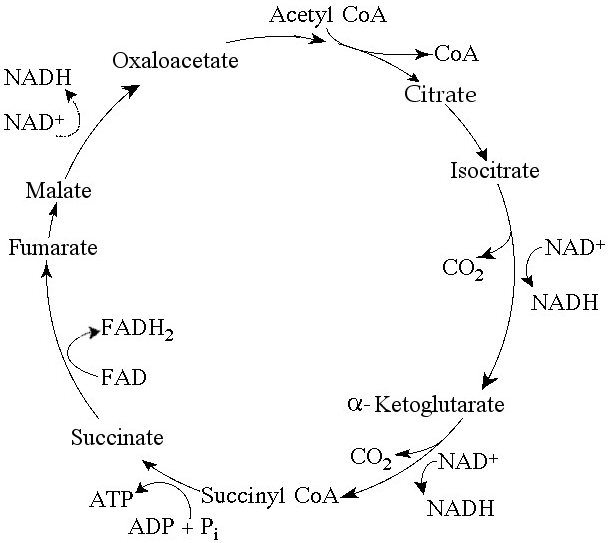
If pyruvate oxidation is blocked, what will happen to the levels of
oxaloacetate and citric acid in the citric acid cycle shown in Figure
9.2?
A) There will be no change in the levels of oxaloacetate and
citric acid.
B) Oxaloacetate will decrease and citric acid will
accumulate.
C) Oxaloacetate will accumulate and citric acid will
decrease.
D) Both oxaloacetate and citric acid will
decrease.
E) Both oxaloacetate and citric acid will accumulate.
Answer: C
Answer: C
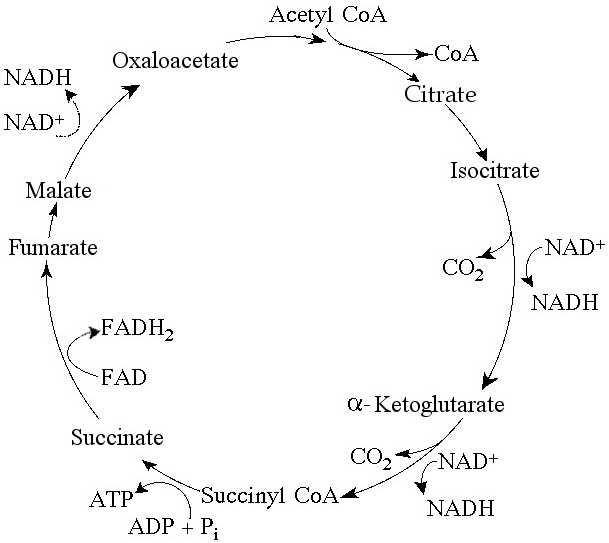
Starting with citrate, which of the following combinations of
products would result from three acetyl CoA molecules entering the
citric acid cycle (see Figure 9.2)?
A) 1 ATP, 2 CO₂, 3 NADH, and
1 FADH₂
B) 2 ATP, 2 CO₂, 3 NADH, and 3 FADH₂
C) 3 ATP, 3
CO₂, 3 NADH, and 3 FADH₂
D) 3 ATP, 6 CO₂, 9 NADH, and 3
FADH₂
E) 38 ATP, 6 CO₂, 3 NADH, and 12 FADH₂
Answer: D
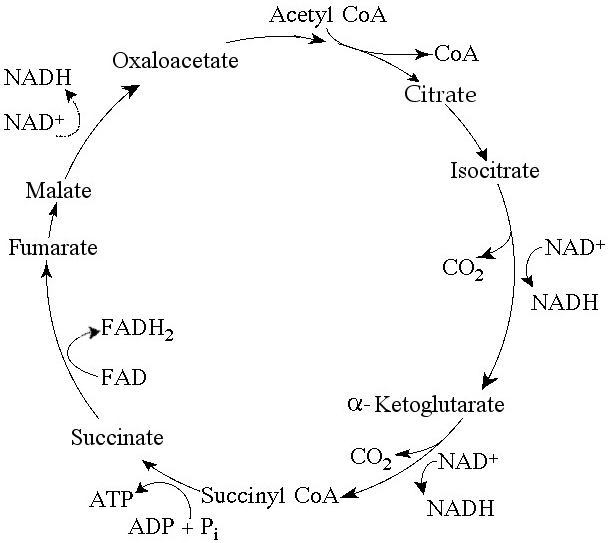
For each molecule of glucose that is metabolized by glycolysis and
the citric acid cycle (see Figure 9.2), what is the total number of
NADH + FADH₂ molecules produced?
A) 4
B) 5
C) 6
D)
10
E) 12
Answer: E
Figure 9.3 shows the electron transport chain. Which of the following
is the combination of substances that is initially added to the
chain?
A) oxygen, carbon dioxide, and water
B) NAD⁺, FAD,
and electrons
C) NADH, FADH₂, and protons
D) NADH, FADH₂,
and O₂
E) oxygen and protons
Answer: D
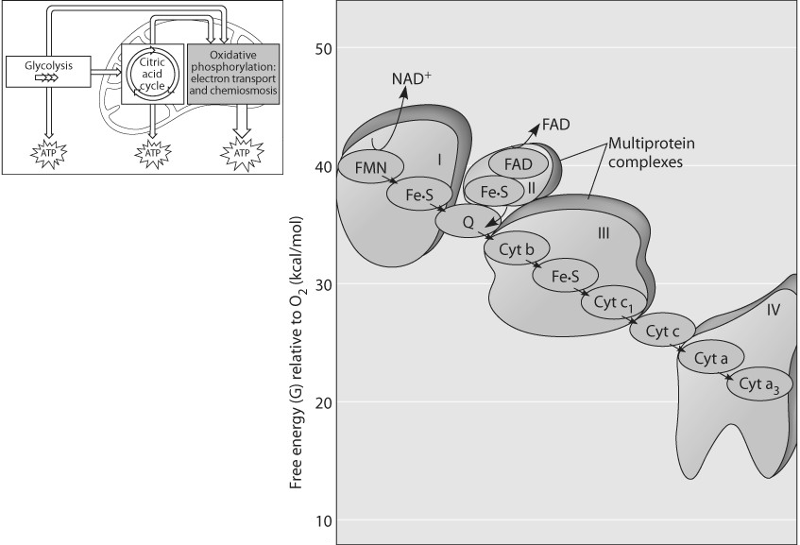
Which of the following most accurately describes what is happening
along the electron transport chain in Figure 9.3?
A) Chemiosmosis
is coupled with electron transfer.
B) Each electron carrier
alternates between being reduced and being oxidized.
C) ATP is
generated at each step.
D) Energy of the electrons increases at
each step.
E) Molecules in the chain give up some of their
potential energy.
Answer: B
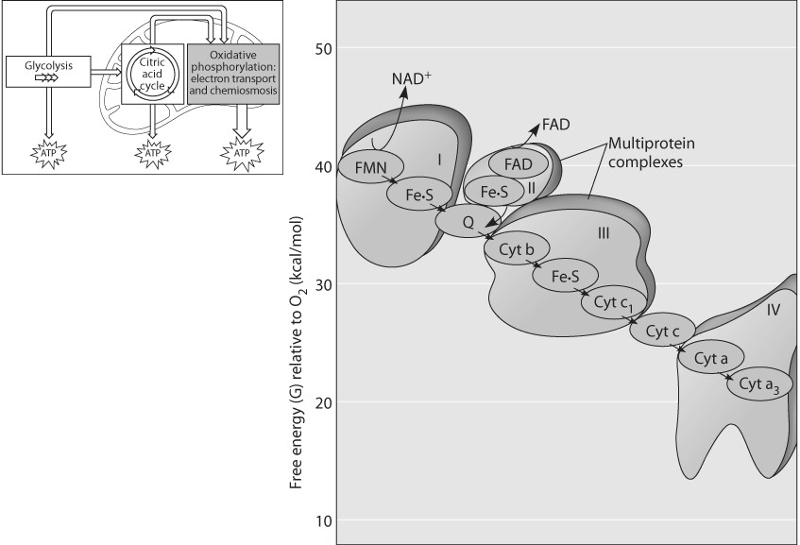
Which of the protein complexes labeled with Roman numerals in Figure
9.3 will transfer electrons to O₂?
A) complex I
B) complex
II
C) complex III
D) complex IV
E) All of the complexes
can transfer electrons to O₂.
Answer: D
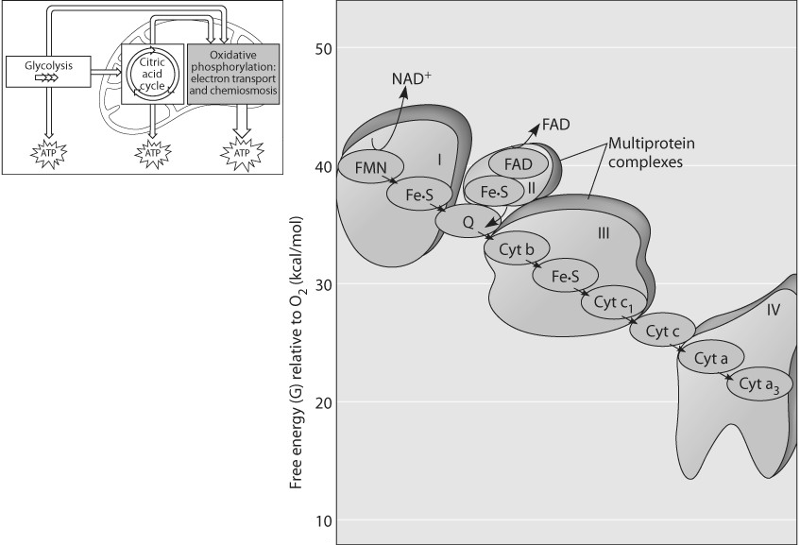
What happens at the end of the chain in Figure 9.3?
A) 2
electrons combine with a proton and a molecule of NAD⁺.
B) 2
electrons combine with a molecule of oxygen and two hydrogen
atoms.
C) 4 electrons combine with a molecule of oxygen and 4
protons.
D) 4 electrons combine with four hydrogen and two oxygen
atoms.
E) 1 electron combines with a molecule of oxygen and a
hydrogen atom.
Answer: C
In the presence of oxygen, the three-carbon compound pyruvate can be
catabolized in the citric acid cycle. First, however, the pyruvate (1)
loses a carbon, which is given off as a molecule of CO₂, (2) is
oxidized to form a two-carbon compound called acetate, and (3) is
bonded to coenzyme A.
These three steps result in the formation of
A) acetyl CoA,
O₂, and ATP.
B) acetyl CoA, FADH₂, and CO₂.
C) acetyl CoA,
FAD, H₂, and CO₂.
D) acetyl CoA, NADH, H⁺, and CO₂.
E)
acetyl CoA, NAD⁺, ATP, and CO₂.
Answer: D
In the presence of oxygen, the three-carbon compound pyruvate can be
catabolized in the citric acid cycle. First, however, the pyruvate (1)
loses a carbon, which is given off as a molecule of CO₂, (2) is
oxidized to form a two-carbon compound called acetate, and (3) is
bonded to coenzyme A.
Why is coenzyme A, a sulfur-containing molecule derived from a B
vitamin, added?
A) because sulfur is needed for the molecule to
enter the mitochondrion
B) in order to utilize this portion of a
B vitamin which would otherwise be a waste product from another
pathway
C) to provide a relatively unstable molecule whose acetyl
portion can be readily transferred to a compound in the citric acid
cycle
D) because it drives the reaction that regenerates
NAD⁺
E) in order to remove one molecule of CO₂
Answer: C
Exposing inner mitochondrial membranes to ultrasonic vibrations will
disrupt the membranes. However, the fragments will reseal "inside
out." These little vesicles that result can still transfer
electrons from NADH to oxygen and synthesize ATP. If the membranes are
agitated further, however, the ability to synthesize ATP is lost.
After the first disruption, when electron transfer and ATP
synthesis still occur, what must be present?
A) all of the
electron transport proteins as well as ATP synthase
B) all of the
electron transport system and the ability to add CoA to acetyl
groups
C) the ATP synthase system
D) the electron transport
system
E) plasma membranes like those bacteria use for respiration
Answer: A
Exposing inner mitochondrial membranes to ultrasonic vibrations will
disrupt the membranes. However, the fragments will reseal "inside
out." These little vesicles that result can still transfer
electrons from NADH to oxygen and synthesize ATP. If the membranes are
agitated further, however, the ability to synthesize ATP is lost.
After the further agitation of the membrane vesicles, what must
be lost from the membrane?
A) the ability of NADH to transfer
electrons to the first acceptor in the electron transport
chain
B) the prosthetic groups like heme from the transport
system
C) cytochromes
D) ATP synthase, in whole or in
part
E) the contact required between inner and outer membrane surfaces
Answer: D
Exposing inner mitochondrial membranes to ultrasonic vibrations will
disrupt the membranes. However, the fragments will reseal "inside
out." These little vesicles that result can still transfer
electrons from NADH to oxygen and synthesize ATP. If the membranes are
agitated further, however, the ability to synthesize ATP is lost.
These inside-out membrane vesicles
A) will become acidic
inside the vesicles when NADH is added.
B) will become alkaline
inside the vesicles when NADH is added.
C) will make ATP from ADP
and i if transferred to a pH 4 buffered solution after incubation in a
pH 7 buffered solution.
D) will hydrolyze ATP to pump protons out
of the interior of the vesicle to the exterior.
E) will reverse
electron flow to generate NADH from NAD⁺ in the absence of oxygen.
Answer: A
The immediate energy source that drives ATP synthesis by ATP synthase
during oxidative phosphorylation is the
A) oxidation of glucose
and other organic compounds.
B) flow of electrons down the
electron transport chain.
C) affinity of oxygen for
electrons.
D) H⁺ concentration across the membrane holding ATP
synthase.
E) transfer of phosphate to ADP.
Answer: D
Which metabolic pathway is common to both fermentation and cellular
respiration of a glucose molecule?
A) the citric acid
cycle
B) the electron transport chain
C) glycolysis
D)
synthesis of acetyl CoA from pyruvate
E) reduction of pyruvate to lactate
Answer: C
In mitochondria, exergonic redox reactions
A) are the source of
energy driving prokaryotic ATP synthesis.
B) are directly coupled
to substrate-level phosphorylation.
C) provide the energy that
establishes the proton gradient.
D) reduce carbon atoms to carbon
dioxide.
E) are coupled via phosphorylated intermediates to
endergonic processes.
Answer: C
The final electron acceptor of the electron transport chain that
functions in aerobic oxidative phosphorylation is
A)
oxygen.
B) water.
C) NAD⁺.
D) pyruvate.
E) ADP.
Answer: A
What is the oxidizing agent in the following reaction?
Pyruvate
+ NADH + H⁺ → Lactate + NAD⁺
A) oxygen
B) NADH
C)
NAD⁺
D) lactate
E) pyruvate
Answer: E
When electrons flow along the electron transport chains of
mitochondria, which of the following changes occurs?
A) The pH of
the matrix increases.
B) ATP synthase pumps protons by active
transport.
C) The electrons gain free energy.
D) The
cytochromes phosphorylate ADP to form ATP.
E) NAD⁺ is oxidized.
Answer: A
Most CO₂ from catabolism is released during
A)
glycolysis.
B) the citric acid cycle.
C) lactate
fermentation.
D) electron transport.
E) oxidative phosphorylation.
Answer: B
If photosynthesizing green algae are provided with CO₂ synthesized
with heavy oxygen (¹⁸O), later analysis will show that all but one of
the following compounds produced by the algae contain the ¹⁸O label.
That one is
A) 3-phosphoglycerate.
B) glyceraldehyde
3-phosphate (G3P).
C) glucose.
D) ribulose bisphosphate
(RuBP).
E) O₂.
Answer: E
Which of the following are products of the light reactions of
photosynthesis that are utilized in the Calvin cycle?
A) CO₂ and
glucose
B) H₂O and O₂
C) ADP, Pi, and NADP⁺
D)
electrons and H⁺
E) ATP and NADPH
Answer: E
Photosynthesis is not responsible for
A) oxygen in the
atmosphere.
B) the ozone layer.
C) most of the organic
carbon on Earth's surface.
D) atmospheric CO₂.
E) fossil fuels.
Answer: E
Where does the Calvin cycle take place?
A) stroma of the
chloroplast
B) thylakoid membrane
C) cytoplasm surrounding
the chloroplast
D) interior of the thylakoid (thylakoid
space)
E) outer membrane of the chloroplast
Answer: A
In any ecosystem, terrestrial or aquatic, what group(s) is (are)
always necessary?
A) autotrophs and heterotrophs
B)
producers and primary consumers
C) photosynthesizers
D)
autotrophs
E) green plants
Answer: D
In autotrophic bacteria, where are the enzymes located that can carry
on carbon fixation (reduction of carbon dioxide to
carbohydrate)?
A) in chloroplast membranes
B) in chloroplast
stroma
C) in the cytosol
D) in the nucleoid
E) in the
infolded plasma membrane
Answer: C
When oxygen is released as a result of photosynthesis, it is a direct
by-product of
A) reducing NADP⁺.
B) splitting water
molecules.
C) chemiosmosis.
D) the electron transfer system
of photosystem I.
E) the electron transfer system of photosystem II.
Answer: B
A plant has a unique photosynthetic pigment. The leaves of this plant
appear to be reddish yellow. What wavelengths of visible light are
being absorbed by this pigment?
A) red and yellow
B) blue
and violet
C) green and yellow
D) blue, green, and
red
E) green, blue, and yellow
Answer: B
Halobacterium has a photosynthetic membrane that is colored purple.
Its photosynthetic action spectrum is exactly complementary (opposite
to) the action spectrum for green plants. What wavelengths of light do
the Halobacterium photosynthetic pigments absorb?
A) red and
yellow
B) blue, green, and red
C) green and yellow
D)
red and green
E) blue and red
Answer: E
In the thylakoid membranes, what is the main role of the antenna
pigment molecules?
A) split water and release oxygen to the
reaction-center chlorophyll
B) harvest photons and transfer light
energy to the reaction-center chlorophyll
C) synthesize ATP from
ADP and Pi
D) transfer electrons to ferredoxin and then
NADPH
E) concentrate photons within the stroma
Answer: B
Which of the events listed below occurs in the light reactions of
photosynthesis?
A) NADP is produced.
B) NADPH is reduced to
NADP⁺.
C) Carbon dioxide is incorporated into PGA.
D) ATP is
phosphorylated to yield ADP.
E) Light is absorbed and funneled to
reaction-center chlorophyll a.
Answer: E
Which statement describes the functioning of photosystem II?
A)
Light energy excites electrons in the thylakoid membrane electron
transport chain.
B) Photons are passed along to a reaction-center
chlorophyll.
C) The P680 chlorophyll donates a pair of protons to
NADP⁺, which is thus converted to NADPH.
D) The electron
vacancies in P680⁺ are filled by electrons derived from water.
E)
The splitting of water yields molecular carbon dioxide as a by-product.
Answer: D
Which of the following are directly associated with photosystem
I?
A) harvesting of light energy by ATP
B) receiving
electrons from the thylakoid membrane electron transport chain
C)
generation of molecular oxygen
D) extraction of hydrogen
electrons from the splitting of water
E) passing electrons to the
thylakoid membrane electron transport chain
Answer: B
Some photosynthetic organisms contain chloroplasts that lack
photosystem II, yet are able to survive. The best way to detect the
lack of photosystem II in these organisms would be
A) to
determine if they have thylakoids in the chloroplasts.
B) to test
for liberation of O₂ in the light.
C) to test for CO₂ fixation in
the dark.
D) to do experiments to generate an action
spectrum.
E) to test for production of either sucrose or starch.
Answer: B
What are the products of linear photophosphorylation?
A) heat
and fluorescence
B) ATP and P700
C) ATP and NADPH
D)
ADP and NADP
E) P700 and P680
Answer: C
As a research scientist, you measure the amount of ATP and NADPH
consumed by the Calvin cycle in 1 hour. You find 30,000 molecules of
ATP consumed, but only 20,000 molecules of NADPH. Where did the extra
ATP molecules come from?
A) photosystem II
B) photosystem
I
C) cyclic electron flow
D) linear electron flow
E) chlorophyll
Answer: C
Assume a thylakoid is somehow punctured so that the interior of the
thylakoid is no longer separated from the stroma. This damage will
have the most direct effect on which of the following
processes?
A) the splitting of water
B) the absorption of
light energy by chlorophyll
C) the flow of electrons from
photosystem II to photosystem I
D) the synthesis of ATP
E)
the reduction of NADP⁺
Answer: D
What does the chemiosmotic process in chloroplasts involve?
A)
establishment of a proton gradient across the thylakoid
membrane
B) diffusion of electrons through the thylakoid
membrane
C) reduction of water to produce ATP energy
D)
movement of water by osmosis into the thylakoid space from the
stroma
E) formation of glucose, using carbon dioxide, NADPH, and ATP
Answer: A
Suppose the interior of the thylakoids of isolated chloroplasts were
made acidic and then transferred in the dark to a pH 8 solution. What
would be likely to happen?
A) The isolated chloroplasts will make
ATP.
B) The Calvin cycle will be activated.
C) Cyclic
photophosphorylation will occur.
D) The isolated chloroplasts
will generate oxygen gas.
E) The isolated chloroplasts will
reduce NADP⁺ to NADPH.
Answer: A
In a plant cell, where are the ATP synthase complexes
located?
A) thylakoid membrane only
B) plasma membrane
only
C) inner mitochondrial membrane only
D) thylakoid
membrane and inner mitochondrial membrane
E) thylakoid membrane
and plasma membrane
Answer: D
In mitochondria, chemiosmosis translocates protons from the matrix
into the intermembrane space, whereas in chloroplasts, chemiosmosis
translocates protons from
A) the stroma to the photosystem
II.
B) the matrix to the stroma.
C) the stroma to the
thylakoid space.
D) the intermembrane space to the
matrix.
E) the thylakoid space to the stroma.
Answer: C
Which of the following statements best describes the relationship
between photosynthesis and respiration?
A) Respiration runs the
biochemical pathways of photosynthesis in reverse.
B)
Photosynthesis stores energy in complex organic molecules, whereas
respiration releases it.
C) Photosynthesis occurs only in plants
and respiration occurs only in animals.
D) ATP molecules are
produced in photosynthesis and used up in respiration.
E)
Respiration is anabolic and photosynthesis is catabolic.
Answer: B
Where are the molecules of the electron transport chain found in
plant cells?
A) thylakoid membranes of chloroplasts
B)
stroma of chloroplasts
C) outer membrane of mitochondria
D)
matrix of mitochondria
E) cytoplasm
Answer: A
In photosynthetic cells, synthesis of ATP by the chemiosmotic
mechanism occurs during
A) photosynthesis only.
B)
respiration only.
C) both photosynthesis and respiration.
D)
neither photosynthesis nor respiration.
E) photorespiration only.
Answer: C
Reduction of oxygen to form water occurs during
A)
photosynthesis only.
B) respiration only.
C) both
photosynthesis and respiration.
D) neither photosynthesis nor
respiration.
E) photorespiration only.
Answer: B
Reduction of NADP⁺ occurs during
A) photosynthesis.
B)
respiration.
C) both photosynthesis and respiration.
D)
neither photosynthesis nor respiration.
E) photorespiration.
Answer: A
The splitting of carbon dioxide to form oxygen gas and carbon
compounds occurs during
A) photosynthesis.
B)
respiration.
C) both photosynthesis and respiration.
D)
neither photosynthesis nor respiration.
E) photorespiration.
Answer: D
Generation of proton gradients across membranes occurs during
A)
photosynthesis.
B) respiration.
C) both photosynthesis and
respiration.
D) neither photosynthesis nor respiration.
E) photorespiration.
Answer: C
What is the relationship between wavelength of light and the quantity
of energy per photon?
A) They have a direct, linear
relationship.
B) They are inversely related.
C) They are
logarithmically related.
D) They are separate phenomena.
E)
They are only related in certain parts of the spectrum.
Answer: B
P680⁺ is said to be the strongest biological oxidizing agent.
Why?
A) It is the receptor for the most excited electron in
either photosystem.
B) It is the molecule that transfers
electrons to plastoquinone (Pq) of the electron transfer
system.
C) It transfers its electrons to reduce NADP⁺ to
NADPH.
D) This molecule has a stronger attraction for electrons
than oxygen, to obtain electrons from water.
E) It has a positive charge.
Answer: D
Some photosynthetic bacteria (e.g., purple sulfur bacteria) have only
photosystem I, whereas others (e.g., cyanobacteria) have both
photosystem I and photosystem II. Which of the following might this
observation imply?
A) Photosystem II was selected against in some
species.
B) Photosynthesis with only photosystem I is more
ancestral.
C) Photosystem II may have evolved to be more
photoprotective.
D) Linear electron flow is more primitive than
cyclic flow of electrons.
E) Cyclic flow is more necessary than
linear electron flow.
Answer: B
electron flow may be photoprotective (protective to light-induced
damage). Which of the following experiments could provide information
on this phenomenon?
A) use mutated organisms that can grow but
that cannot carry out cyclic flow of electrons and compare their
abilities to photosynthesize in different light intensities against
those of wild-type organisms
B) use plants that can carry out
both linear and cyclic electron flow, or only one or another of these
processes, and compare their light absorbance at different wavelengths
and different light intensities
C) use bacteria that have only
cyclic flow and look for their frequency of mutation damage at
different light intensities
D) use bacteria with only cyclic flow
and measure the number and types of photosynthetic pigments they have
in their membranes
E) use plants with only photosystem I
operative and measure how much damage occurs at different wavelengths
Answer: A
Carotenoids are often found in foods that are considered to have
antioxidant properties in human nutrition. What related function do
they have in plants?
A) They serve as accessory pigments to
increase light absorption.
B) They protect against oxidative
damage from excessive light energy.
C) They shield the sensitive
chromosomes of the plant from harmful ultraviolet radiation.
D)
They reflect orange light and enhance red light absorption by
chlorophyll.
E) They take up and remove toxins from the groundwater.
Answer: B
In thylakoids, protons travel through ATP synthase from the thylakoid
space to the stroma. Therefore, the catalytic "knobs" of ATP
synthase would be located
A) on the side facing the thylakoid
space.
B) on the ATP molecules themselves.
C) on the pigment
molecules of photosystem I and photosystem II.
D) on the stromal
side of the membrane.
E) built into the center of the thylakoid
stack (granum).
Answer: D
In metabolic processes of cell respiration and photosynthesis,
prosthetic groups such as heme and iron-sulfur complexes are
encountered in components of the electron transport chain. What do
they do?
A) donate electrons
B) act as reducing
agents
C) act as oxidizing agents
D) transport protons
within the mitochondria and chloroplasts
E) both oxidize and
reduce during electron transport
Answer: E
In a cyanobacterium, the reactions that produce NADPH occur
in
A) the light reactions alone.
B) the Calvin cycle
alone.
C) both the light reactions and the Calvin cycle.
D)
neither the light reactions nor the Calvin cycle.
E) the
chloroplast, but is not part of photosynthesis.
Answer: A
The reactions that produce molecular oxygen (O₂) take place
in
A) the light reactions alone.
B) the Calvin cycle
alone.
C) both the light reactions and the Calvin cycle.
D)
neither the light reactions nor the Calvin cycle.
E) the
chloroplast, but are not part of photosynthesis
Answer: A
The accumulation of free oxygen in Earth's atmosphere began
A)
with the origin of life and respiratory metabolism.
B) with the
origin of photosynthetic bacteria that had photosystem I.
C) with
the origin of cyanobacteria that had both photosystem I and
photosystem II.
D) with the origin of chloroplasts in
photosynthetic eukaryotic algae.
E) with the origin of land plants.
Answer: C
A flask containing photosynthetic green algae and a control flask
containing water with no algae are both placed under a bank of lights,
which are set to cycle between 12 hours of light and 12 hours of dark.
The dissolved oxygen concentrations in both flasks are monitored.
Predict what the relative dissolved oxygen concentrations will be in
the flask with algae compared to the control flask.
A) The
dissolved oxygen in the flask with algae will always be
higher.
B) The dissolved oxygen in the flask with algae will
always be lower.
C) The dissolved oxygen in the flask with algae
will be higher in the light, but the same in the dark.
D) The
dissolved oxygen in the flask with algae will be higher in the light,
but lower in the dark.
E) The dissolved oxygen in the flask with
algae will not be different from the control flask at any time.
Answer: D
Where do the enzymatic reactions of the Calvin cycle take
place?
A) stroma of the chloroplast
B) thylakoid
membranes
C) matrix of the mitochondria
D) cytosol around
the chloroplast
E) thylakoid space
Answer: A
What is the primary function of the Calvin cycle?
A) use ATP to
release carbon dioxide
B) use NADPH to release carbon
dioxide
C) split water and release oxygen
D) transport RuBP
out of the chloroplast
E) synthesize simple sugars from carbon dioxide
Answer: E
In C₃ photosynthesis, the reactions that require ATP take place
in
A) the light reactions alone.
B) the Calvin cycle
alone.
C) both the light reactions and the Calvin cycle.
D)
neither the light reactions nor the Calvin cycle.
E) the
chloroplast, but is not part of photosynthesis.
Answer: B
In a plant leaf, the reactions that produce NADH occur in
A) the
light reactions alone.
B) the Calvin cycle alone.
C) both
the light reactions and the Calvin cycle.
D) neither the light
reactions nor the Calvin cycle.
E) the chloroplast, but is not
part of photosynthesis.
Answer: D
The NADPH required for the Calvin cycle comes from
A) reactions
initiated in photosystem I.
B) reactions initiated in photosystem
II.
C) the citric acid cycle.
D) glycolysis.
E)
oxidative phosphorylation.
Answer: A
Reactions that require CO₂ take place in
A) the light reactions
alone.
B) the Calvin cycle alone.
C) both the light
reactions and the Calvin cycle.
D) neither the light reactions
nor the Calvin cycle.
E) the chloroplast, but is not part of photosynthesis.
Answer: B
Which of the following statements best represents the relationships
between the light reactions and the Calvin cycle?
A) The light
reactions provide ATP and NADPH to the Calvin cycle, and the cycle
returns ADP, Pi, and NADP⁺ to the light reactions.
B) The light
reactions provide ATP and NADPH to the carbon fixation step of the
Calvin cycle, and the cycle provides water and electrons to the light
reactions.
C) The light reactions supply the Calvin cycle with
CO₂ to produce sugars, and the Calvin cycle supplies the light
reactions with sugars to produce ATP.
D) The light reactions
provide the Calvin cycle with oxygen for electron flow, and the Calvin
cycle provides the light reactions with water to split.
E) There
is no relationship between the light reactions and the Calvin cycle.
Answer: A
Three "turns" of the Calvin cycle generate a
"surplus" molecule of glyceraldehyde 3-phosphate (G3P).
Which of the following is a consequence of this?
A) Formation of
a molecule of glucose would require nine "turns."
B)
G3P more readily forms sucrose and other disaccharides than it does
monosaccharides.
C) Some plants would not taste sweet to
us.
D) The formation of sucrose and starch in plants involves
assembling G3P molecules, with or without further
rearrangements.
E) Plants accumulate and store G3P.
Answer: D
In the process of carbon fixation, RuBP attaches a CO₂ to produce a
six-carbon molecule, which is then split to produce two molecules of
3-phosphoglycerate. After phosphorylation and reduction produces
glyceraldehyde 3-phosphate (G3P), what more needs to happen to
complete the Calvin cycle?
A) addition of a pair of electrons
from NADPH
B) inactivation of RuBP carboxylase enzyme
C)
regeneration of ATP from ADP
D) regeneration of RuBP
E)
regeneration of NADP⁺
Answer: D
The pH of the inner thylakoid space has been measured, as have the pH
of the stroma and of the cytosol of a particular plant cell. Which, if
any, relationship would you expect to find?
A) The pH within the
thylakoid is less than that of the stroma.
B) The pH of the
stroma is lower than that of the other two measurements.
C) The
pH of the stroma is higher than that of the thylakoid space but lower
than that of the cytosol.
D) The pH of the thylakoid space is
higher than that anywhere else in the cell.
E) There is no
consistent relationship.
Answer: A
The phylogenetic distribution of the enzyme rubisco is limited
to
A) C₃ plants only.
B) C₃ and C₄ plants.
C) all
photosynthetic eukaryotes.
D) all known photoautotrophs, both
bacterial and eukaryotic.
E) all living cells.
Answer: D
Photorespiration occurs when rubisco reacts RuBP with
A)
CO₂.
B) O₂.
C) glyceraldehyde 3-phosphate.
D)
3-phosphoglycerate.
E) NADPH.
Answer: B
In an experiment studying photosynthesis performed during the day,
you provide a plant with radioactive carbon (¹⁴C) dioxide as a
metabolic tracer. The ¹⁴C is incorporated first into oxaloacetate. The
plant is best characterized as a
A) C₄ plant.
B) C₃
plant.
C) CAM plant.
D) heterotroph.
E) chemoautotroph.
Answer: A
Why are C₄ plants able to photosynthesize with no apparent
photorespiration?
A) They do not participate in the Calvin
cycle.
B) They use PEP carboxylase to initially fix CO₂.
C)
They are adapted to cold, wet climates.
D) They conserve water
more efficiently.
E) They exclude oxygen from their tissues.
Answer: B
CAM plants keep stomata closed in daytime, thus reducing loss of
water. They can do this because they
A) fix CO₂ into organic
acids during the night.
B) fix CO₂ into sugars in the
bundle-sheath cells.
C) fix CO₂ into pyruvate in the mesophyll
cells.
D) use the enzyme phosphofructokinase, which outcompetes
rubisco for CO₂.
E) use photosystem I and photosystem II at night.
Answer: A
Photorespiration lowers the efficiency of photosynthesis by
A)
carbon dioxide molecules.
B) 3-phosphoglycerate
molecules.
C) ATP molecules.
D) ribulose bisphosphate
molecules.
E) RuBP carboxylase molecules.
Answer: B
The alternative pathways of photosynthesis using the C₄ or CAM
systems are said to be compromises. Why?
A) Each one minimizes
both water loss and rate of photosynthesis.
B) C₄ compromises on
water loss and CAM compromises on photorespiration.
C) Both
minimize photorespiration but expend more ATP during carbon
fixation.
D) CAM plants allow more water loss, while C₄ plants
allow less CO₂ into the plant.
E) C₄ plants allow less water loss
but CAM plants allow more water loss.
Answer: C
If plant gene alterations cause the plants to be deficient in
photorespiration, what would most probably occur?
A)
Photosynthetic efficiency would be reduced at low light
intensities.
B) Cells would carry on the Calvin cycle at a much
slower rate.
C) Less ATP would be generated.
D) There would
be more light-induced damage to the cells.
E) Less oxygen would
be produced.
Answer: D
Compared to C₃ plants, C₄ plants
A) can continue to fix CO₂ even
at relatively low CO2 concentrations and high oxygen
concentrations.
B) have higher rates of photorespiration.
C)
do not use rubisco for carbon fixation.
D) grow better under
cool, moist conditions.
E) make a four-carbon compound,
oxaloacetate, which is then delivered to the citric acid cycle in mitochondria.
Answer: A
If atmospheric CO₂ concentrations increase twofold or more, how will plants be affected, disregarding any changes in climate?
A) All plants will experience increased rates of photosynthesis.
B) C₃ plants will have faster growth; C₄ plants will be minimally affected.
C) C₄ plants will have faster growth; C₃ plants will be minimally affected.
D) C₃ plants will have faster growth; C₄ plants will have slower growth.
E) Plant growth will not be affected because atmospheric CO₂ concentrations are never limiting for plant growth.
Answer: B
Plants photosynthesize only in the light. Plants respire
A) in
the dark only.
B) in the light only.
C) both in light and
dark.
D) never–they get their ATP from
photophosphorylation.
E) only when excessive light energy induces photorespiration.
Answer: C
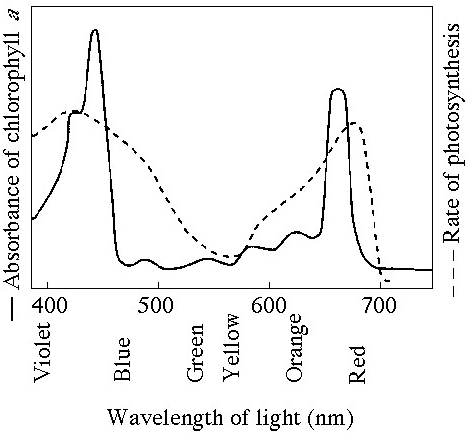
Figure 10.1 shows the absorption spectrum for chlorophyll a and the
action spectrum for photosynthesis. Why are they different?
A)
Green and yellow wavelengths inhibit the absorption of red and blue
wavelengths.
B) Bright sunlight destroys photosynthetic
pigments.
C) Oxygen given off during photosynthesis interferes
with the absorption of light.
D) Other pigments absorb light in
addition to chlorophyll a.
E) Aerobic bacteria take up oxygen,
which changes the measurement of the rate of photosynthesis.
Answer: D
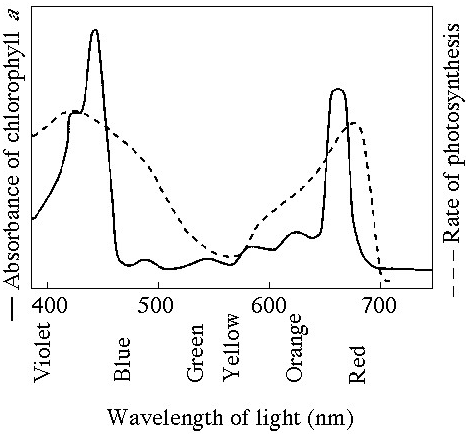
What wavelength of light in the figure is most effective in driving
photosynthesis?
A) 420 mm
B) 475 mm
C) 575 mm
D)
625 mm
E) 730 mm
Answer: A
If ATP used by this plant is labeled with radioactive phosphorus,
which molecule or molecules of the Calvin cycle will be radioactively
labeled first?
A) B only
B) B and C only
C) B, C, and D
only
D) B and E only
E) B, C, D, and E
Answer: D
If the carbon atom of the incoming CO₂ molecule is labeled with a
radioactive isotope of carbon, which organic molecules will be
radioactively labeled after one cycle?
A) C only
B) B, C, D,
and E
C) C, D, and E only
D) B and C only
E) B and D only
Answer: B
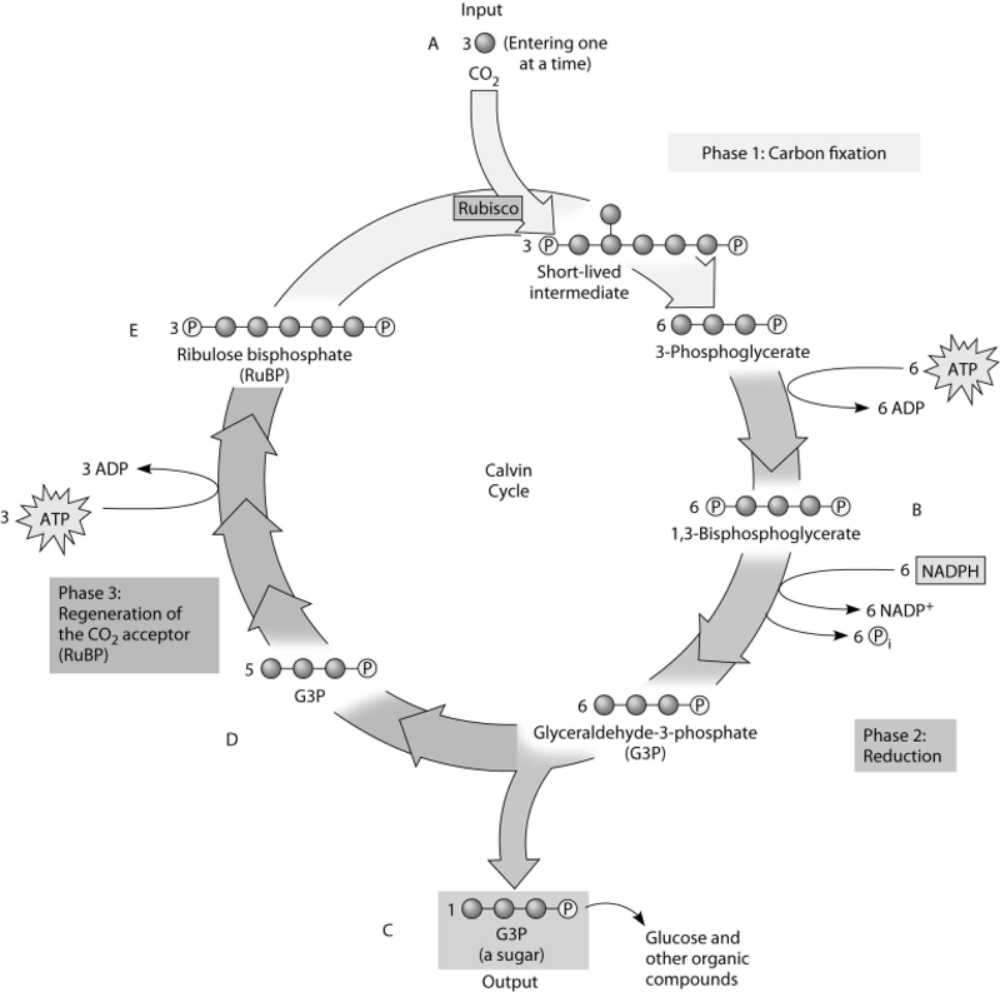
Which molecule(s) of the Calvin cycle is (are) also found in
glycolysis?
A) B, C, E, and 3-phosphoglycerate
B) B, C, and
E only
C) 3-phosphoglycerate only
D) B, C, D, and
3-phosphoglycerate only
E) E only
Answer: D
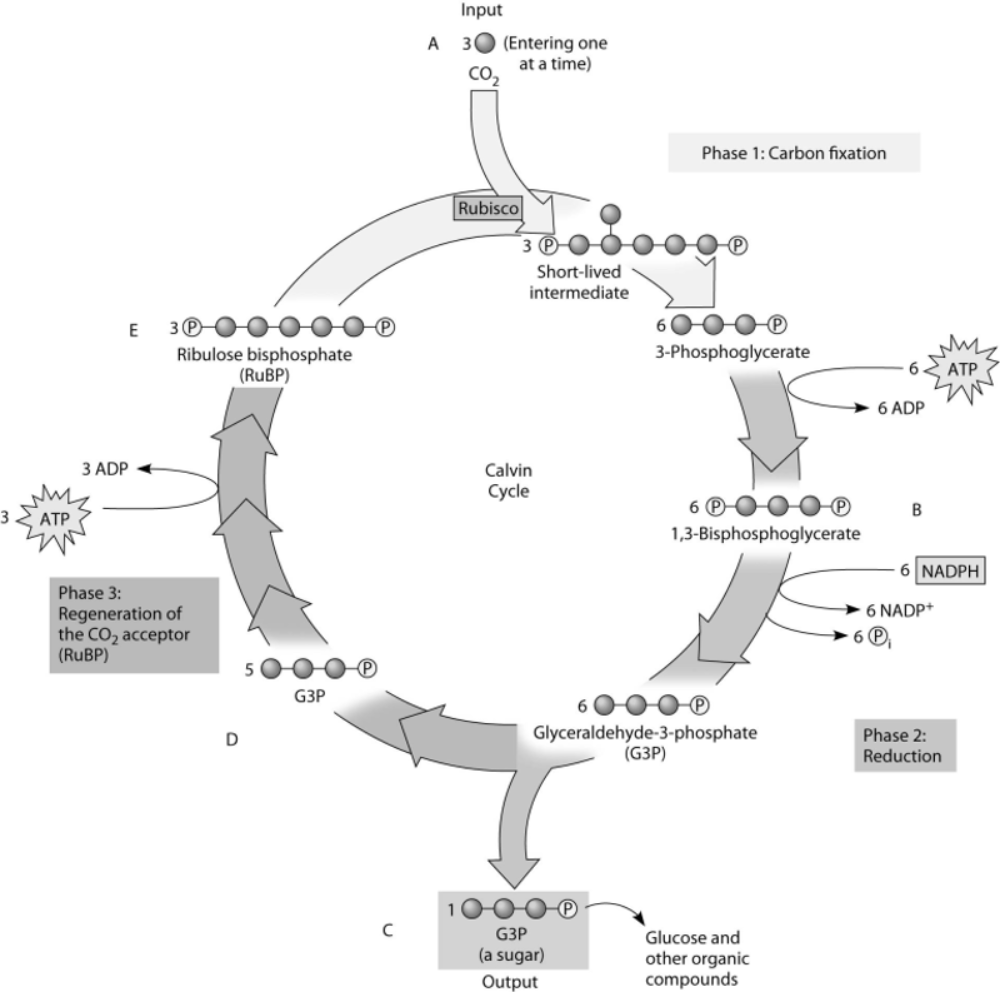
To identify the molecule that accepts CO₂, Calvin and Benson
manipulated the carbon-fixation cycle by either cutting off CO₂ or
cutting off light from cultures of photosynthetic algae. They then
measured the concentrations of various metabolites immediately
following the manipulation. How would these experiments help identify
the CO₂ acceptor? Study Figure 10.2 to help you in determining the
correct answer.
A) The CO₂ acceptor concentration would decrease
when either the CO₂ or light are cut off.
B) The CO₂ acceptor
concentration would increase when either the CO₂ or light are cut
off.
C) The CO₂ acceptor concentration would increase when the
CO₂ is cut off, but decrease when the light is cut off.
D) The
CO₂ acceptor concentration would decrease when the CO₂ is cut off, but
increase when the light is cut off.
E) The CO₂ acceptor
concentration would stay the same regardless of the CO₂ or light.
Answer: C
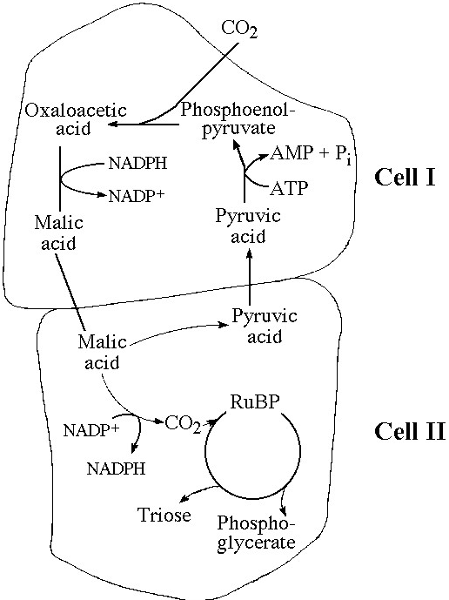
Which of the following statements is true concerning Figure
10.3?
A) It represents cell processes involved in C₄
photosynthesis.
B) It represents the type of cell structures
found in CAM plants.
C) It represents an adaptation that
maximizes photorespiration.
D) It represents a C₃ photosynthetic
system.
E) It represents a relationship between plant cells that
photosynthesize and those that cannot.
Answer: A
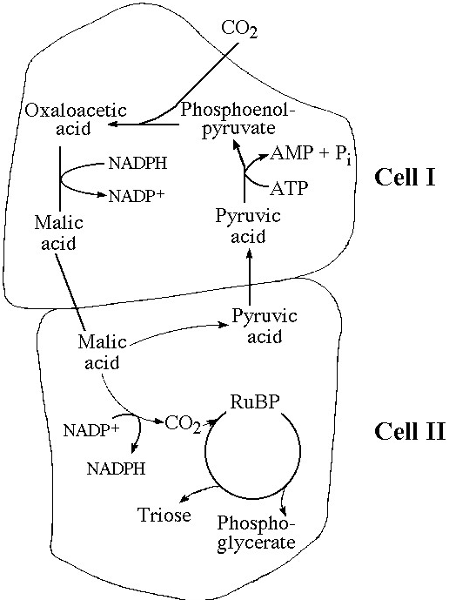
Referring to Figure 10.3, oxygen would inhibit the CO₂ fixation
reactions in
A) cell I only.
B) cell II only.
C)
neither cell I nor cell II.
D) both cell I and cell II.
E)
cell I during the night and cell II during the day.
Answer: B
A gardener is concerned that her greenhouse is getting too hot from
too much light, and seeks to shade her plants with colored translucent
plastic sheets. What color should she use to reduce overall light
energy, but still maximize plant growth?
A) green
B)
blue
C) yellow
D) orange
E) any color will work equally well
Answer: B
Theodor W. Engelmann illuminated a filament of algae with light that
passed through a prism, thus exposing different segments of algae to
different wavelengths of light. He added aerobic bacteria and then
noted in which areas the bacteria congregated. He noted that the
largest groups were found in the areas illuminated by the red and blue light.
What did Engelmann conclude about the congregation of bacteria
in the red and blue areas?
A) Bacteria released excess carbon
dioxide in these areas.
B) Bacteria congregated in these areas
due to an increase in the temperature of the red and blue
light.
C) Bacteria congregated in these areas because these areas
had the most oxygen being released.
D) Bacteria are attracted to
red and blue light and thus these wavelengths are more reactive than
other wavelengths.
E) Bacteria congregated in these areas due to
an increase in the temperature caused by an increase in photosynthesis.
Answer: C
Theodor W. Engelmann illuminated a filament of algae with light that
passed through a prism, thus exposing different segments of algae to
different wavelengths of light. He added aerobic bacteria and then
noted in which areas the bacteria congregated. He noted that the
largest groups were found in the areas illuminated by the red and blue light.
An outcome of this experiment was to help determine
A) the
relationship between heterotrophic and autotrophic organisms.
B)
the relationship between wavelengths of light and the rate of aerobic
respiration.
C) the relationship between wavelengths of light and
the amount of heat released.
D) the relationship between
wavelengths of light and the rate of photosynthesis.
E) the
relationship between the concentration of carbon dioxide and the rate
of photosynthesis.
Answer: D
Theodor W. Engelmann illuminated a filament of algae with light that
passed through a prism, thus exposing different segments of algae to
different wavelengths of light. He added aerobic bacteria and then
noted in which areas the bacteria congregated. He noted that the
largest groups were found in the areas illuminated by the red and blue light.
If you ran the same experiment without passing light through a
prism, what would you predict?
A) There would be no difference in
results.
B) The bacteria would be relatively evenly distributed
along the algal filaments.
C) The number of bacteria present
would decrease due to an increase in the carbon dioxide
concentration.
D) The number of bacteria present would increase
due to an increase in the carbon dioxide concentration.
E) The
number of bacteria would decrease due to a decrease in the temperature
of the water.
Answer: B
A spaceship is designed to support animal life for a multiyear voyage
to the outer planets of the solar system. Plants will be grown to
provide oxygen and to recycle carbon dioxide.
Since the spaceship will be too far from the sun for
photosynthesis, an artificial light source will be needed. What
wavelengths of light should be used to maximize plant growth with a
minimum of energy expenditure?
A) full-spectrum white
light
B) green light
C) a mixture of blue and red
light
D) yellow light
E) UV light
Answer: C
A spaceship is designed to support animal life for a multiyear voyage
to the outer planets of the solar system. Plants will be grown to
provide oxygen and to recycle carbon dioxide.
If the power fails and the lights go dark, what will happen to
CO₂ levels?
A) CO₂ will rise as a result of both animal and plant
respiration.
B) CO₂ will rise as a result of animal respiration
only.
C) CO₂ will remain balanced because plants will continue to
fix CO₂ in the dark.
D) CO₂ will fall because plants will
increase CO₂ fixation.
E) CO₂ will fall because plants will cease
to respire in the dark.
Answer: A
The light reactions of photosynthesis supply the Calvin cycle
with
A) light energy.
B) CO₂ and ATP.
C) H₂O and
NADPH.
D) ATP and NADPH.
E) sugar and O₂.
Answer: D
Which of the following sequences correctly represents the flow of
electrons during photosynthesis?
A) NADPH → O₂ → CO₂
B) H₂O
→ NADPH → Calvin cycle
C) NADPH → chlorophyll → Calvin
cycle
D) H₂O → photosystem I → photosystem II
E) NADPH →
electron transport chain → O₂
Answer: B
How is photosynthesis similar in C₄ plants and CAM plants?
A) In
both cases, only photosystem I is used.
B) Both types of plants
make sugar without the Calvin cycle.
C) In both cases, rubisco is
not used to fix carbon initially.
D) Both types of plants make
most of their sugar in the dark.
E) In both cases, thylakoids are
not involved in photosynthesis.
Answer: C
Which of the following statements is a correct distinction between
autotrophs and heterotrophs?
A) Only heterotrophs require
chemical compounds from the environment.
B) Cellular respiration
is unique to heterotrophs.
C) Only heterotrophs have
mitochondria.
D) Autotrophs, but not heterotrophs, can nourish
themselves beginning with CO₂ and other nutrients that are
inorganic.
E) Only heterotrophs require oxygen.
Answer: D
Which of the following does not occur during the Calvin
cycle?
A) carbon fixation
B) oxidation of NADPH
C)
release of oxygen
D) regeneration of the CO₂ acceptor
E)
consumption of ATP
Answer: C
In mechanism, photophosphorylation is most similar to
A)
substrate-level phosphorylation in glycolysis.
B) oxidative
phosphorylation in cellular respiration.
C) the Calvin
cycle.
D) carbon fixation.
E) reduction of NADP⁺.
Answer: B
Which process is most directly driven by light energy?
A)
creation of a pH gradient by pumping protons across the thylakoid
membrane
B) carbon fixation in the stroma
C) reduction of
NADP⁺ molecules
D) removal of electrons from chlorophyll
molecules
E) ATP synthesis
Answer: D